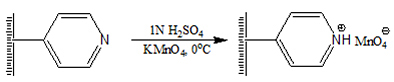|
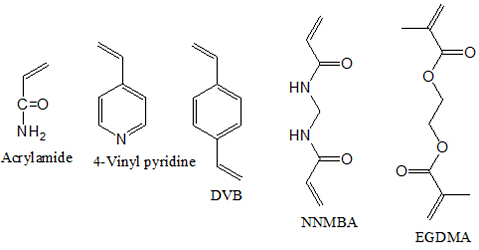
Introduction The chemistry of polymer-supported reagents has been an active field of research in recent years [1-3]. A polymeric-supported organic reagent can provide an effective alternative to its low molecular weight counterpart. Nowadays, the environmental and economic pressure is forcing the chemical community to search for more efficient ways of performing chemical transformations in a single operation by reusable catalysts without toxic and costly reagents. Reactions using solid-supported reagents and scavengers have created considerable interest among synthetic chemists around the world [4-6]. Major advantages of polymer-supported reagents are the possibility of reducing the potential of pollution stemming from chemical research and industrial chemical processes, potential for automated synthesis and testing using high-throughput screens, simplified product isolation, and recovery, the use of excess reagent to drive reactions to completion and can be easily recycled and reused [7-9]. Due to several advantages in terms of yield, purity, and selectivity, supported reagents have been used under solvent-free conditions for the synthesis of various important synthetic intermediates. Another attractive feature is that the polymeric reagent is non-volatile and the reduced volatility, increases the shelf life of the reagent so that, the supported analog can be stored under ordinary conditions for a long period. Polymer-supported reagents have been developed for use in functional group transformations, in peptide synthesis, as stoichiometric reagents, and catalysts, in ion-exchange chromatography and immobilization of enzymes [10-13]. Oxidation is an important class of reactions from both the industrial and academic points of view. Polymer-supported oxidizing agents have attracted the attention of many scientists due to their various unique features and have been used for a variety of industrial reactions [14-17]. Polymer-supported hypobromites, iodates, periodates, and permanganate were reported as efficient recyclable oxidizing agents [18, 19]. In organic chemistry, potassium permanganate is widely used as one of the most versatile of the commonly used oxidants. Oxidation of organic substrates with low molecular weight potassium permanganate poses many problems. Most organic solvents are either readily oxidized by potassium permanganate or they do not dissolve the permanganate ion in appreciable amounts. These difficulties led to the use of heterogeneous conditions for permanganate oxidation. The present paper is based on the development of a new class of various cross-linked poly(4-vinylpyridine) based polymers, their synthetic applications and studies on the role of the polymer support on the reactivity of this reagent. The main advantage of cross-linked polymer-supported solid-phase organic reagents over their monomeric counterparts in organic synthesis is the ease of separation of the excess reagent from the desired reaction product. Experimental Materials 4-vinylpyridine, acrylamide, ethylene glycol dimethacrylate, N, N'-methylene-bis-acrylamide, and divinylbenzene were commercial products availed from Aldrich Chemical Company, USA. All low molecular weight compounds were commercially available samples and were purified by distillation under reduced pressure immediately before use or recrystallization unless otherwise stated. IR spectra were recorded on Shimdzu FT-IR-8400 S spectrophotometer. UV spectra were recorded on Shimdzu UV-2450 spectrophotometer. Melting points were measured using Buchi-530 melting point apparatus. Methods Preparation of 5-20 mol% DVB-, EGDMA- and NNMBA- cross-linked poly[4-vinyl(pyridine-co-acrylamide)s] based permanganates For the preparation of 5-20 mol% DVB-, EGDMA- and NNMBA-cross-linked poly[4-vinyl(pyridine-co-acrylamide)s], the monomers 4-vinylpyridine (5.1228 ml), acrylamide (3.3763 g) and required an amount of the crosslinking agent was dissolved in methanol (20 ml). The initiator AIBN (100 mg) was added and the mixture heated to 60ºC and stirred. Heating and stirring continued for 6 h. It was cooled to room temperature. The product was filtered, washed with water, acetone, dichloromethane and finally with methanol. Incorporation of permanganate Function into cross-linked polymers: General procedure To a suspension of the cross-linked polymer (10 g) in water (40 ml), 1N H2SO4 (10 ml) was added and stirred for 1 hour. A solution of KMnO4 (12 g) in water (100 ml) containing 1N H2SO4 (10 ml) was added to the suspension with vigorous stirring at 0ºC. The mixture was stirred for 1 h at 0ºC and 2 h at room temperature. The product was filtered at the pump and washed with water until the filtrate was completely free from permanganate ions. The polymer was then washed with a little methanol and dried in vacuum to yield a dark powder. Estimation of permanganate in the functionalized polymers: General procedure A definite amount of (0.1 g) of the functionalized polymer was accurately weighed and suspended in excess of 2N H2SO4 (10 ml) for 1 h. A known excess of standard ferrous ammonium sulfate solution was added and stirred until the dark color of the polymer disappeared. The unreacted ferrous ammonium sulfate solution was titrated against the standard KMnO4 solution. From the titer value, the permanganate equivalent was calculated. Oxidation reaction using polymer-supported permanganates: General procedure To a five-fold molar excess of the reagent moistened with water, a solution of the substrate in a suitable solvent (20 ml) was added and stirred at refluxing temperature of the solvent. The progress of the reaction was followed by TLC. After 24 h, the reaction mixture was filtered and the polymer was washed with solvent. The filtrate was dried over anhydrous sodium sulfate and the product was isolated by evaporating the solvent. Oxidation of benzoin to benzyl was carried out to find out the dependence of temperature, solvent, molar excess of the polymeric reagent and time duration on oxidation reaction. Investigation of the effect of the reaction condition on the extent of oxidation In order to investigate the dependence of various parameters like solvent, molar excess of the reagent, temperature and time on the oxidation by polymer-supported permanganates, benzoin to benzil oxidation was taken as a model reaction. a. Effect of solvent To study the effect of various solvents on the oxidation, the oxidation of benzoin to benzil was carried out in solvents of varying polarity. Five-fold molar excess of the reagent was used and oxidations were carried out at refluxing temperature of the solvent. In each case, the extent of oxidation was followed by TLC. The various solvents used were chloroform, cyclohexane, dioxane-water, and dichloromethane. b. Effect of molar excess of the reagent A calculated quantity of the reagent (for the appropriate molar excess based on capacity) was weighed and made wet with water added to benzoin (100 mg) dissolved in cyclohexane and refluxed. The extent of oxidation was followed by TLC. c. Effect of temperature Five-fold molar excess of the reagent was added to benzoin (100 mg) in cyclohexane at room temperature (30ºC) and the extent of oxidation was followed by TLC. The same experiment was repeated at temperature 50ºC and refluxing temperature of the solvent. d. Effect of time Five-fold molar excess of the reagent was added to benzoin (100 mg) in cyclohexane and refluxed. The extent of oxidation was followed by TLC at regular intervals of time. Recycling and Reuse The spent polymeric reagent was stirred for 2 h with 2 N (50 ml) of ferrous ammonium sulfate in the presence of 2 N H2SO4 (20 ml) to remove all oxides of manganese. The polymer was filtered, washed with water and methanol, and dried. The regenerated polymer was functionalized as mentioned earlier. The recycled polymer was used for oxidations as mentioned earlier. Results & Discussion In the present study oxidation behavior of permanganate functions supported on cross-linked poly(4-vinylpyridine-co-acrylamide) are detailed along with the dependence of oxidation behavior on the nature of polymer support and nature of crosslinking. Preparation of cross-linked poly[4-vinyl(pyridine-co-acrylamide)]-type supports Cross-linked poly[4-vinyl(pyridine-co-acrylamide)] based supports were prepared by free radical initialized solution polymerization using AIBN as the initiator. The monomers and crosslinking agents used are given in Scheme 1. The polymer was then functionalized with permanganate to afford a dark powder (Scheme 2).
The permanganate capacity of the new functionalized polymer was determined by excess-back titration with standard ferrous ammonium sulfate solution in the presence of dilute sulphuric acid. Permanganate capacities of various cross-linked poly(4-vinylpyridine) based polymers are shown in Table 1. The FT-IR spectrum of the permanganate incorporated polymers showed intense absorptions at 420, 790, 820 and 900 cm-1. The absorption at 900 cm-1 is characteristic of the permanganate ion.
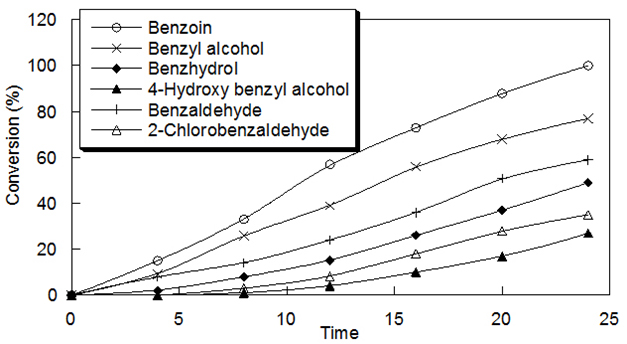
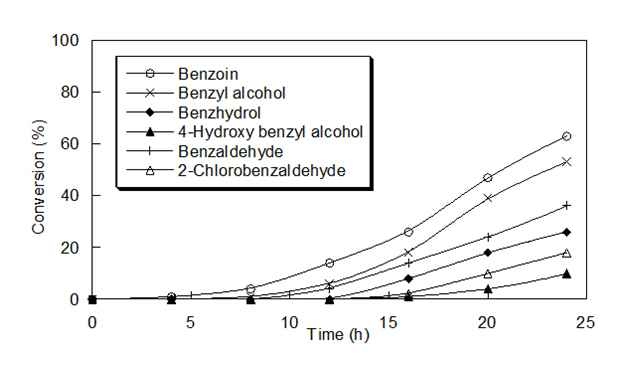
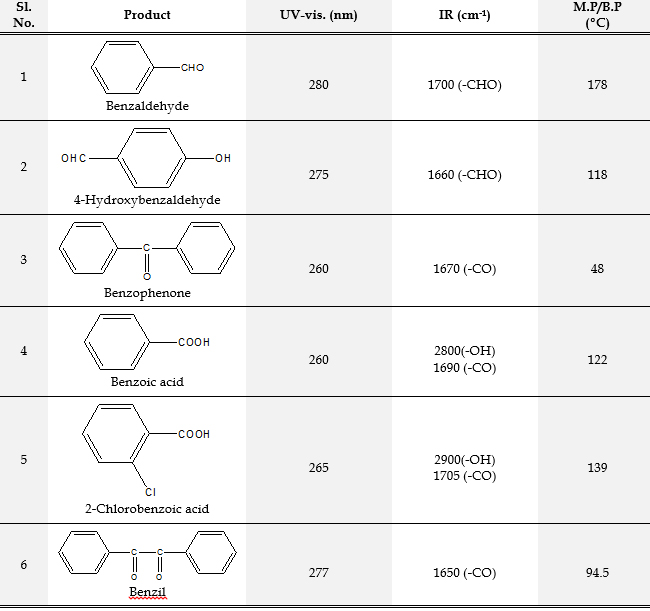
Oxidations Reactions Using The oxidizing ability of poly(4-vinylpyridine-co-acrylamide)-permanganate polymers was investigated in detail for its ability to oxidize alcohols to carbonyl compounds. Primary alcohols were converted to aldehydes, secondary alcohols to ketones and aldehydes to corresponding acids. The details of oxidation reactions of different alcohols and aldehydes with the polymer are
given in Table 2. The oxidation conditions involve stirring of the reaction mixture in cyclohexane and refluxed for the indicated period. In all these cases of oxidation wetting of the polymer with water was found to be necessary. The progress of the reaction was followed by TLC. After 24 h, the reaction mixture was filtered and the polymer was washed with solvent. The filtrate was dried over anhydrous sodium sulfate and the product was isolated by evaporating the solvent. The products were characterized by melting point/boiling point, IR, and UV-vis. Spectra.
Effect of Various Parameters on Oxidation Reaction In order to investigate the dependence of various parameters like solvent, temperature, and molar excess of the reagent on oxidation benzoin to benzil was carried out as a model reaction.
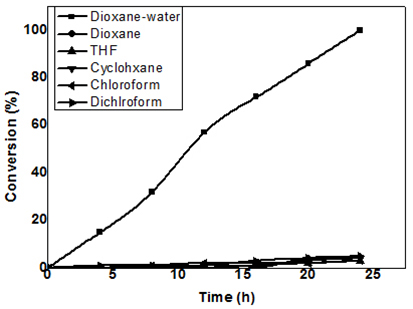
Effect of Solvent The oxidation reactions were investigated in solvents of different polarity like dioxane, tetrahydrofuran, chloroform, cyclohexane, dichloromethane and dioxane-water mixture (1:1). Because of the hydrophilic nature of the acrylamide, the reagent could not swell in any other solvent than dioxane: water mixture (1:1). Hence all oxidations were carried out in dioxane: water mixture.
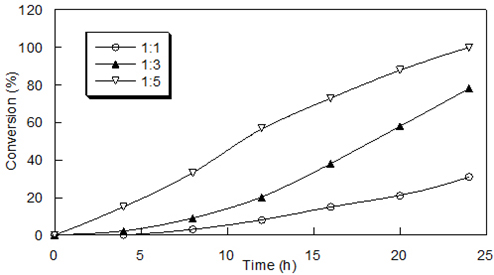
Effect of Molar Excess of Reagent Oxidation of benzoin was carried out by varying the molar excess of poly[4-vinyl(pyridine-co-acrylamide)]-permanganate, keeping other experimental conditions the same. The oxidation reactions of 1 mmol of benzoin with a different molar excess of polymeric reagent are studied. With increasing substrate to reagent ratio, the extent of oxidation increased. The results are presented in Figure 5.
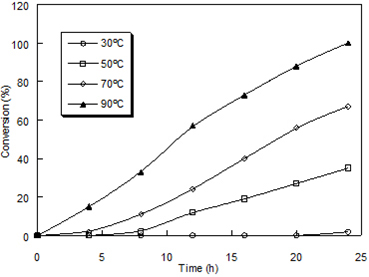
Effect of temperature Temperature is one of the major factors influencing the rate of the polymer-supported oxidation reaction. To study the effect of temperature on the reactivity of poly[4-vinyl(pyridine-co-acrylamide)]-permanganate, benzoin to benzil oxidation was conducted at various temperatures ranging from room temperature to 90ºC. Figure 6 shows the extent of conversion increased as the temperature increased.
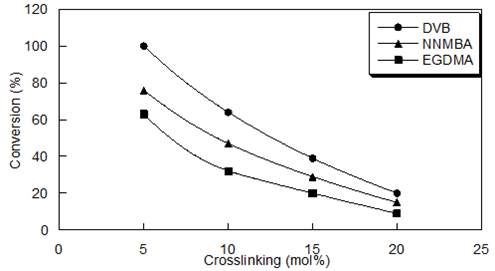
Effect of the nature and degree of crosslinking on the reactivity of various cross-linked poly[4-vinyl(pyridine-co-acrylamide)]-permanganates In order to investigate the oxidizing ability of permanganate functions supported on 5–20 mol% DVB-, NNMBA- and EGDMA-cross-linked poly[4-vinyl(pyridine-co-acrylamide)], oxidation of benzoin to benzil, was selected as a model reaction. As shown in Figure 7, in all cases the 5 mol% cross-linked system has high reactivity and decreased further with increasing crosslinking. The observation of the decrease of the rate of oxidation of functionalized polymer with an increase in crosslink density is due to the decreased availability of the reactive sites buried within the crosslinks. For an effective oxidation to occur, the functional group should be more accessible to the reagent in the solvent phase. As the degree of crosslinking increases, the availability of the reactive moiety decreases even in the presence of good solvents.
The nature of the crosslinking agent does influence the kinetics and extent of functional group conversions in polymer-aided reactions. Thus in the present case, the reactivity decreased in the order: DVB- > NNMBA- > EGDMA-cross-linked system. Even though the permanganate capacity of the NNMBA-cross-linked system is high its low reactivity than the DVB-cross-linked system is due to the non-compatibility between the hydrophilic support and the hydrophobic substrate in the dioxin-water medium. The oxidizing ability of permanganate functions supported poly (4-vinylpyridine) is higher than the corresponding poly[4-vinyl(pyridine-co-acrylamide)]. This is due to the extreme hydrophilicity and inter-chain hydrogen bonding of the amide groups present in the copolymer. This hinders the swelling of the polymer matrix in organic solvents from hydrophilic-hydrophobic imbalance resulting in lower reactivity. The dioxane-water mixture, the best solvent for the copolymer is not a favorable medium for oxidation. Recycling & Reuse of the Spent Reagent An important advantage of the polymeric reagent over their low molecular weight counterpart is that the polymer bound reagent could be regenerated and reused. The regeneration was achieved by removing all the manganese oxides by treating with acidic ferrous ammonium sulfate solution in Table 3. The recycling process was repeated several times without appreciable loss of activity, and the resulting polymer could be used for subsequent oxidation reactions.
Conclusions Permanganate functions supported on cross-linked poly(4-vinylpyridine-co-acrylamide) polymers could oxidize primary and secondary alcohols to aldehydes and ketones, and aldehydes to corresponding carboxylic acids. Among the various cross-linked systems, the oxidation capacity decreased in the order: DVB > NNMBA >EGDMA. Even though the permanganate capacity of the NNMBA-cross-linked system is high, it has a lower reactivity than the DVB-cross-linked system is due to the non-compatibility between the hydrophilic support and the hydrophobic substrate in the dioxane-water medium. The observation of the decrease in the rate of oxidation of functionalized polymer with an increase in crosslink density is due to the decreased availability of the reactive sites within the crosslinks. The various reaction parameters already as noted were investigated. The suitable solvent for this polymer is dioxane: water (1:1) due to the hydrophilic nature of the acrylamide. The yield of the product increases with increasing temperature. Polymeric reagent could be used in excess to get a good yield without causing any separation problem and over oxidation products. It is observed that a high molar excess of polymeric reagent resulted in an enhanced rate of reaction. References
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||