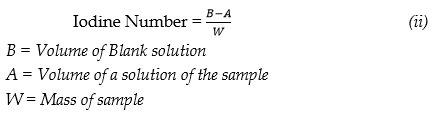|
Introduction The presence of inorganic and organic pollutants in wastewater is increasing and several of them are not efficiently removed by conventional wastewater treatment processes and pose re-occurring and persistent problems such as odor, toxicity, and foaming. Several studies have been conducted on the use of plant and animal materials for the removal of heavy metals from aqueous solution by adsorption [1-11]. Activated carbons and modified natural materials have been found to be excellent adsorbents materials, and thus are used to purify, decolorize, detoxify, filter or remove dissolved substances. They are also used as catalysts in industrial processes [12]. Activated carbon has long been serving the industrial need of decolorizing and removal of unwanted dissolved impurities from aqueous and non-aqueous media [13]. Activated carbons made from conventional raw materials such as coconut shell, bituminous coal, peat, and lignite, however, are expensive [14]. Activated carbons are prepared by physical and chemical activation methods. There are two important advantages of chemical activation over physical activation. Lowering of temperature, and the yield of the chemical activation [15]. Acid activation followed by the thermal treatment increases the adsorption capacity to a large extent due to the increased surface area and pore volume [16, 17]. Many researchers have worked on the production of adsorbents materials from renewable resources using low-cost methods and materials, with emphasis to decontaminate water in the environment. Agricultural and industrial waste material is used as adsorbents by different researchers for the removal of heavy metals, such as coconut husk, palm pressed fibers, coconut shell activated carbon, wood-dust coal activated carbons [18-20], and used rubber tire carbon [21]. Cactus, olive stone, wool, charcoal and pine needles [22], rice husk carbon [12], Cequisetifolia leaf carbon [22], bagasse and fly ash [23], hazelnut shell [24], husk of Bengal gram [25], sugarcane bagasse [15], rice bran [26], and rice husk (Hindu) have been reported in literature. Jatropha curcas is a multi-purpose non-edible oil yielding perennial shrub and a drought tolerant plant, with their seeds used to produce renewable energy such as bio-diesel. Indian railways use 2 million kg diesel per year. Indian Government has decided to use bio-diesel at 5% level in the regular diesel. It has started planting Jatropha along both sides of the railway tracts, which will cover an area of 2500 km2. Many researchers grow these plants for biodiesel production supported by several funding agencies. This investigation addresses the preparation of a number of adsorbents using different acids, such as citric acid and orthophosphoric acid. All the chemicals used are of analytical reagent grade. Experimental Materials and Methods The root, stem and seed coat of Jatropha Curcas was obtained from Agbo-oba area in Ilorin, Kwara State, Nigeria. The root, stem, and seed coat of the Jatropha curcas plant were chopped into smaller sizes and exposed to outdoor sunlight (drying) for about 2 days. The dried samples were then divided into two portions for the modification and carbonization processes. The portion for modification was ground to a smaller size using mortar and pestle. Preparations Citric Acid Modified Samples: The air dried and ground samples were soaked in excess 0.3 M HNO3 for 24 hrs at room temperature. The mixture was purified with de-ionized water until pH of washing mixture was 6.8. The crystals were filtered and air dried with the samples steeped in 0.6 M citric acid overnight. The citric acid modified crystals were severally surface cleaned with distilled water to remove excess citric acid, and finally air dried and kept in a closed container [13]. Activated Carbon: The dried roots, stem and seed coat from the second portion were carbonized each separately in a muffle furnace at a temperature of 450o C for 4hrs. The carbonized samples were ground and further sieved to 150 μm. The filtered charcoal was treated with 75% orthophosphoric acid (H3PO4) at a ratio of 1:1 by weight of charcoal-H3PO4. The mixture of H3PO4 and charcoal were dried in an oven for 24 hrs at The citric acid modified samples and carbonized samples were labeled as shown in Table 1.
Ash Content: Sample ash content was performed by weighing, 1 g MDS, MDR, MDC, ACS, ACR, and ACC. These were placed in a crucible of known mass and heated in a furnace (Fisher-Thermo Scientific, USA.) at a temperature of 600o C for 4 hrs. The heated samples were placed into desiccators for cooling and reweighed. The ash content of each sample was calculated from the weight of the sample before and after heating [29].
pH: 1 g each of MDS, MDR MDC, ACS, ACR, and ACC was boiled in a beaker containing 100 ml of distilled water for 5 min. These solutions were diluted to 200 ml with distilled water and cooled to room temperature. The corresponding measured pH was performed using a pH meter (model ATPH-6, USA.) [30]. Iodine Number: The iodine number (iodine value) of each of the prepared samples was determined by adding 1 g of the sample to 20 ml of 5% HCl and boiled. The solution was allowed to be cooled down to room temperature. 100 ml of 0.1 N Iodine solution was added and stirred for 30 min using a magnetic stirrer; the solution was filtered and 20 ml each of filtrates was titrated with sodium thiosulphate (Na2S2O3) solution in the presence of a starch indicator. The iodine value of each sample was determined from the reading of titration containing the sample and that of the blank [31]. Blank titration using 0.1 N iodine solution prepared was titrated with 0.1 N Na2S2O3 and the readings obtained were used as the blank.
Moisture Content: Samples of MDS, MDR MDC, ACS, ACR, and ACC were placed into the crucible of known mass and placed inside an oven of 80oC for 3 hrs. Samples were removed and cooled over silica in a desiccator and weighed and reheated at an oven temperature of 105oC for an additional 30 mins. This process was repeated 5 - 7 times for 15 minutes until a constant mass was achieved. The percentage moisture contents of each sample were calculated from the readings obtained [29].
Bulk Density: The bulk densities of each of samples were determined by using Archimedes’ principle. This was determined by weighing an empty measuring cylinder, versus the mass of cylinder containing each sample, versus the mass of cylinder filled with water to the brim. The mass of each sample was determined from the difference in mass of the filled and empty measuring cylinder. The volume of water in the container was determined by taking the difference in mass of the empty and filled measuring cylinder. The bulk densities were determined by dividing the mass of each by the volume of the measuring cylinder [32].
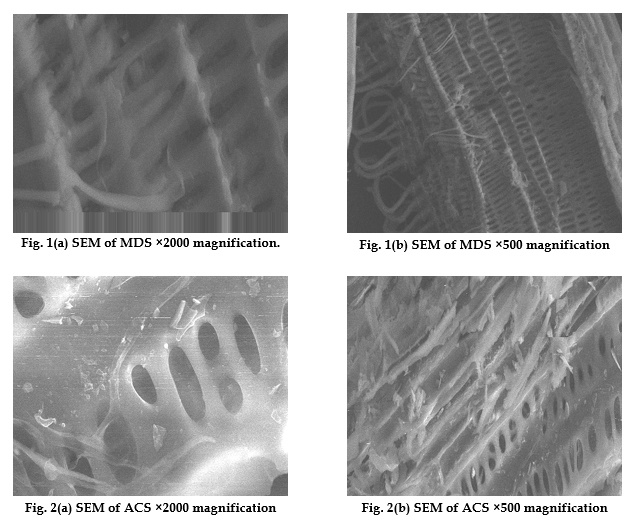
Results & Discussion Scanning Electron Microscopy The surface characteristics of MDS, MDR, MDC, ACS, ACR and ACC were analyzed using scanning electron microscopy in order to visualize the samples surface morphology by magnifying their pore sizes, using The JSM-7400F (USA) field emission scanning electron microscope having a 1 nm resolution at 15 kV and a 1.5 nm resolution at 1 kV. This allows operation in a wide range of beam energies from 0.1 keV to 30 keV and affords various operation modes, including secondary electron imaging (SEI), backscattered electron imaging, gentle beam mode, and imaging with energy filters. The surface morphology of MDS, MDC, MDR, ACR, ACS, and ACC was visualized via scanning electron microscopy (SEM), the corresponding SEM micrographs were obtained at ×2000 (2 kx) and ×500 magnifications. Photographic images of SEM micrographs of MDS, MDC, MDR, ACR, ACS, and ACC showed spongy or coarse areas of the surface of the carbon and the microspores were identifiable. It is generally understood that pore structures development is influenced by many factors, such as inorganic impurities, and the initial structure of the carbon precursor. For the SEM images of MDS and ACS, see Figures 1 (a-b) and 2 (a-b), respectively. The surfaces showed relatively smooth regions as well as cracked and pitted morphology. The presence of small pores on the surface indicated that the adsorbents had developed an elementary pore network. It can be shown that chemical activation resulted in a porous structure opening of the sample surfaces of MDS and ACS. According to the International Union of Pure and Applied Chemistry (IUPAC) [33] pores on activated carbons are classified by their sizes into three groups: macropores having an average diameter > 50 nm, mesopores with a diameter of 2–50 nm, and micropores having an average diameter of < 2 nm.
Physicochemical Properties of the Samples The moisture content, which is the amount of water present in a sample, suggests that extensive porosity has been introduced during the acid treatment in the structure of all carbons. The moisture content of the samples was found to be 4.2, 4.7, 5.1, 4.8, 5.6, and 5.2% [Table 2]. These do not affect the adsorptive power of the sample. It was observed that if the moisture content of adsorbent is high, it will limit the action of the samples and necessitate utilizing some extra loads of the carbon [34]. Similar characteristics are observed in the moisture content of Ricinus Communis,found to be 2.5 [29], and the moisture content of Jatropha Husk activated with H3PO4 is 15 [35], which indicated that the result obtained was consistent with the same data found in other studies. Sample data obtained from this study is tabulated in Table 2. The iodine number is a relative indicator of porosity in a carbonaceous material and may be used as an approximation of surface area for some types of carbon; higher iodine numbers reflect the better development of the microporous structure and higher adsorption abilities for low-molar-mass solutes [36, 37]. The iodine number of the samples MDS, MDR, MDC, ACS, ACR, and ACC was 244.9, 208.5, 237.3, 233.1, 229.6 and 232.5, respectively [Table 2]. This is consistent with the results obtained in Jatropha Husk activated with H3PO4, HCI, ZnCl2, NaOH, H2SO4 and steam (81, 157, 91, 138, 301 and 112) [35]. These indicate that the sample prepared by acid activation has a good adsorptive capacity and can be considered for adsorption of heavy metals and organic pollutants. Iodine number of samples are usually high at a temperature of 400oC because of the incomplete carbonization process at 400oC. The tar left over also contributed to iodine absorption [38]. It was clear that the iodine number of ACR, ACS and ACC is high due to the phosphoric acid destroying the aliphatic and aromatic species present in plants, therefore swiftly removing the volatile matter during the carbonization process [39]. The ash content generally provides information about the inorganic constituents associated with carbon. Ash content of adsorbents usually increases with an increase in carbonization temperature. This was believed to be due to reduced volatile matter (VM). The percentage ash contents of MDS, MDC, MDR, ACR, ACS, and ACC is 7.8, 8.4, 3.6, 7.4, 8.3 and 8.2% [Table 2]. The ash content of adsorbents increased with a decrease in percentage of volatile matter, which indicates that ash is non-volatile [40]. The percentage ash content of Jatropha Husk activated by NaOH, H2SO4, H3PO4, HCl, ZnCl2, and steam were 7.4, 12.5, 10.2, 7.2, 0.5, 31.8% [35]; another report indicates the percentage ash content of Ricinus Communis as 6.5 [33]. All of these indicate that the ash content of MDS, MDC, MDR, ACR, ACS, and ACC is in the normal range [41, 42]. The pH of adsorbent is the degree of acidity or basicity of that adsorbent and it depends on the methods of preparation, inorganic matter and chemically active oxygen groups on its surface, as well as the kind of treatment to which the adsorbent had undergone [43]. The pH of the solution after mixing MDS, MDC, MDR, ACR, ACS, and ACC with distilled water is 6.5, 6.8, 6.7, 6.8, 6.7 and 6.6, respectively. These results correspond to the results of Jatropha Husk activated by NaOH, H2SO4, H3PO4, HCl, ZnCl2 and steam, which are 7.8, 6.98, 6.74, 6.33, 6.01 and 8.11 [35], and Ricinus Communis which is 6.90 [29]. These data indicate that the adsorption of H+ ions from solution or desorption of OH- ions from the sorbent surface [44]. Bulk density is an important parameter when carbon is removed by filtration. It determines how many pounds of carbons can be contained in a filter of a given solid capacity and how much treated liquid is retained by the cake filtrate. Carbons with adequate density also help to improve the filtration rate by forming an even cake on the filter surface. The American Water Work Association has set a lower limit on bulk density at 0.25 gm/ml for Granular Activated Carbon (GACs) to be of practical use [26, 33]. The bulk density of the prepared sample used for this work is within that limit, which is 0.52 gm/ml. The bulk densities of MDS, MDC, MDR, ACR, ACS and ACC were 0.109, 0.120, 0.224, 0.152, 0.21 and 0.37 g/m3. These results imply that the outcome in this experiment corresponds well with the expected and standard value.
Elemental Analysis of MDS, MDC, MDR, ACR, ACS, and ACC The XRF results of MDS, MDC, MDR, ACR, ACS, and ACC showed that K, Ca, Fe and Zn are the major elements present, while Sr is also high in MDS, respectively (as seen in Table 3). Other elements present are either too low at ultra-trace levels or not detected. This shows that MDS, MDC, MDR, ACR, ACS, and ACC cannot be used as adsorbents in the removal of metals like K, Ca, Fe and Zn, as well as I, which is a non-metal due to the adsorption process taking place, leading to an increase in the amount of these elements instead of their removal. Conclusions Preparation of adsorbents from pyrolysis and modification of physic nut plant was performed on a laboratory-scale. Data obtained indicate that the adsorbents with favorable physicochemical properties may be produced using several methods. A number of adsorbents were prepared by activation with acids such as ZnCl2, H3PO4, and NaOH, and characterized using standard methods. The MDS, MDR, MDC, ACS, ACR, ACC in this study has shown to have excellent physicochemical properties and the SEM showed they had constructed or organized surface morphology structures. The iodine number of the adsorbents is in the range 244.9, 208.5 - 244.9, which is in agreement with the result obtained in Jatropha Husk activation with H3PO4. These showed that they could be used as effective adsorbents for the removal of contaminants from water. As the raw material of the carbons is disposed of as waste, applications of the MDS, MDR, MDC, ACS, ACR, ACC adsorbents to the water treatments are expected to be low cost and effective. Acknowledgment The authors thank the Center for Hydrogen Storage and Research Laboratory at Department of Chemistry, Delaware State University, Delaware, USA, Department of Chemistry at the University of Ilorin, Kwara State, Nigeria, and Department of Environmental Science and Policy laboratory at Wilmington University, New Castle, Delaware, USA, for their supportive roles. References
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||