|
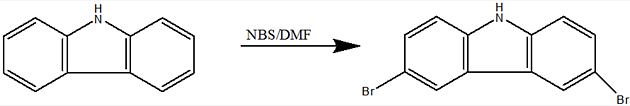
Introduction With the recent increase in awareness of environmental and energy issues, renewable energy sources are given more and more attention. Solar energy provides clean abundant energy and is, therefore, an excellent candidate for a future environmentally friendly energy source. The main aim of solar cell research is to increase the solar energy conversion efficiency at low cost to provide a cost-effective sustainable energy source. Photovoltaic cells are devices that convert the incident photon energy of the solar radiation into electrical energy. Looking at photovoltaics, a dye-sensitized solar cell is considered to be a promising candidate; they have a long shelf life and are low-cost renewable energy sources [1]. Organic materials can be chemically tuned to adjust to physical properties such as band gap and conduction energies, charge transport, solubility, and morphological properties. Small quantities are needed for device preparation. Organic photovoltaic solar cells, therefore, have the potential for development in the search for low-cost modules for the production of domestic electricity [2]. Basic work in our laboratory includes the synthesis of hole transporting materials for the fabrication of solid-state dye-sensitized solar cells [3]. Carbazole based derivatives have attracted much attention because of their interesting photochemical properties [4]. Another fascinating advantage is the versatility of the carbazole reactive sites that can be substituted with a wide variety of functional groups, allowing fine-tuning of its optical and electrical properties [5]. The carbazole derivatives generally possess good thermal stability and hole transport properties [6]. The combination of carbazole derivatives and triphenylamine derivatives is expected to offer improved thermal and morphological stabilities as well as their good hole transport properties [7]. Molecules contain a π-rich heterocyclic or aromatic ring system functionalized with one or more electron donating substituents exhibits good hole transporting properties. The most commonly encountered substituents are amino and alkoxy groups, which contain single bonded heteroatoms possessing sharable lone pairs. These molecules are easily oxidized to resonance stabilized radical cations, which are the actual positive charge bearing species. The most widely used hole transport molecule are aromatic amines, such as anilines, diphenylamines, triphenylamines, carbazoles, and their derivatives. Carbazole based compounds have interesting photochemical properties. Recent interest in the carbazole derivatives has been caused by its good charge transport function, which can be exploited in the molecular design of new types of HTMS in DSCs [8-9]. The present work focuses on the synthesis of hole transporting materials based on carbazole with triphenylamine derivatives. Carbazole based material constitute a well-known class of hole conducting material. The charge carrier mobility and photoconductive properties of these materials have been studied by various groups. Hole transporting materials based on the carbazole moiety have been the subject of an increasing number of investigations over the last decade. This could be explained by the very interesting features such as low cost of the starting material, good chemical and environmental stability provided by the fully aromatic unit, easy substitution of the nitrogen atom with a wide range of functional groups permitting better solubility and fine tuning of the electronic and optical properties [10-13]. Triphenylamine cored star-burst materials are extensively investigated for their easy modification, superior hole transporting ability and propeller molecular conformation [14]. Triphenylamines were also used as the core to construct an optoelectronic functional star-burst material [15]. Triarylamine based compounds find increasing importance as hole transporting materials in various electro-optical applications like photovoltaic, light emitting devices and photorefractive systems. Triarylamine moiety fulfills the requirement of easy and reversible oxidation and therefore constitutes the building block of many of the hole transporting compounds [16]. Various derivatives of triarylamine have been successfully applied in solid-state dye-sensitized TiO2 solar cells [17]. Experimental Materials Infrared (IR) spectra were recorded on a Shimadzu FT-IR 8400 S spectrometer as Potassium bromide (KBr) disc. Ultraviolet-Visible (UV-Vis) spectra of a dilute solution in spectroscopic grade chloroform were recorded on a UV-Vis Shimadzu 1700 using 1.0 cm length quartz tube.1H and 13 C nuclear magnetic resonance (NMR) spectra were recorded on an NMR-JEOL GSX-400 spectrometer with tetramethylsilane as the internal reference using CDCl3 as the solvent in all cases. Mass spectra were recorded on JEOL GCMATE II GC-MS. Methods Synthesis of 3, 6 – dibromocarbazole (A) Carbazole 1.67 g (0.01 mol) was dissolved in 15 ml DMF at 0°C with stirring followed by the addition of a solution of NBS 3.63 g (0.02 mol) in 10 ml of DMF. The resulting mixture was stirred at room temp for 2 hr and the solution then poured into 100 ml of water, filtered and washed with water. The crude product was recrystallized from ethanol. Yield: 64%, Appearance: White crystalline solid, Melting point: 204°C. The completion of the reaction was monitored by TLC.
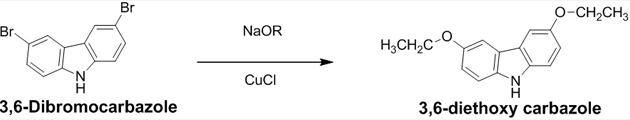
Synthesis of 3, 6 – diethoxycarbazole (B) Into a three-necked flask fitted with a condenser, N2 inlet, addition funnel, and magnetic stirrer were added 25 ml of dry ethanol. The entire solution was cooled to 0°C with ice water before sodium 2.3 g (0.1 mol) was added gradually. The ice bath was removed and the mixture was stirred until all the sodium had reacted. To this, sodium ethoxide solution was added DMF 12.5 ml, CuCl 3.8 g (0.03 mol), 3, 6-dibromocarbazole 1.65 g (0.005 mol) and another 12.5 ml of DMF. The resulting mixture was heated to reflux for 4 hrs under nitrogen. The precipitate was filtered while hot and the filtrate was diluted with 50 ml of water and extracted with chloroform (30 ml x 3). The combined organic layers were neutralized with 5% HCl followed by washing with water and brine, drying by passing through sodium sulphate and concentrating in the vacuum. The residue was recrystallized from methanol. Yield: 60%, Appearance: Pale brown solid, Melting point: 185°C.
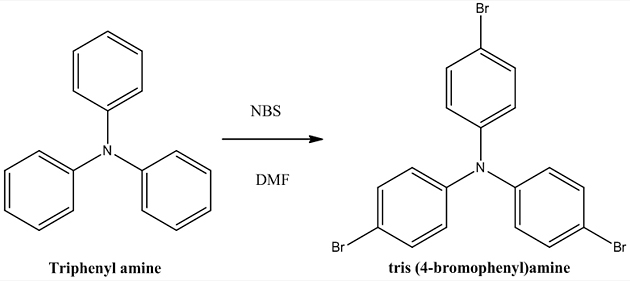
Synthesis of tris (4-bromo phenyl) amine (C) Triphenylamine 2.45 g (0.01mol) was dissolved in 20 ml DMF at 0°C with stirring followed by the addition of a solution of NBS 5.34 g (0.03 mol) in 10 ml of DMF. The resulting mixture was stirred at room temp for 2 hr and the solution then poured into 100 ml of water, filtered and washed with water. The crude product was recrystallized from methanol. Yield: 62%, Appearance: Pale yellow solid, Melting point: 141°C.
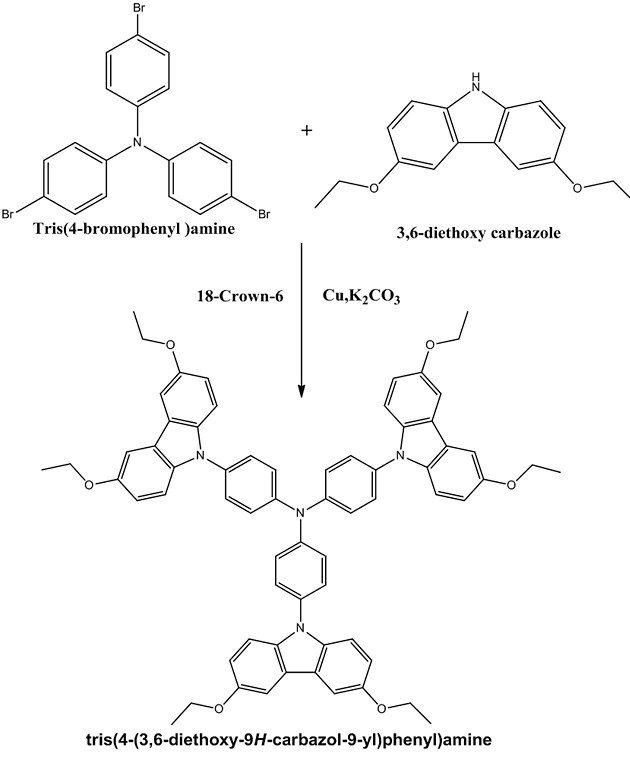
Synthesis of tris(4-(3,6-diethoxy-9H-Carbazol-9 yl) phenyl)amine (D) 3,6-Diethoxycarbazole 0.7658 g (0.003 mol), tris (4-bromophenyl)amine 0.4820 g (0.001 mol), K2CO3 2.65 g(0.02 mol), Copper powder1.8 g (0.03 mol) and 18-crown-6 200 mg(0.00075 mol) were heated together in 50 ml of orthodichlorobenzene under nitrogen atmosphere and the resulting mixture was refluxed for 24 hrs at 170°C. The inorganic compounds were removed by filtration and the filtrate was diluted with water. The combined organic layers washed with brine, dried by passing through sodium sulphate and concentrating in vacuum to give the brown residue which was purified by chromatographic column using ethyl acetate-hexane (3:1) as the eluent to obtain brown solid. Recrystallized from acetone. Yield: 54%, Appearance: Brown solid, Melting point: 160°C.
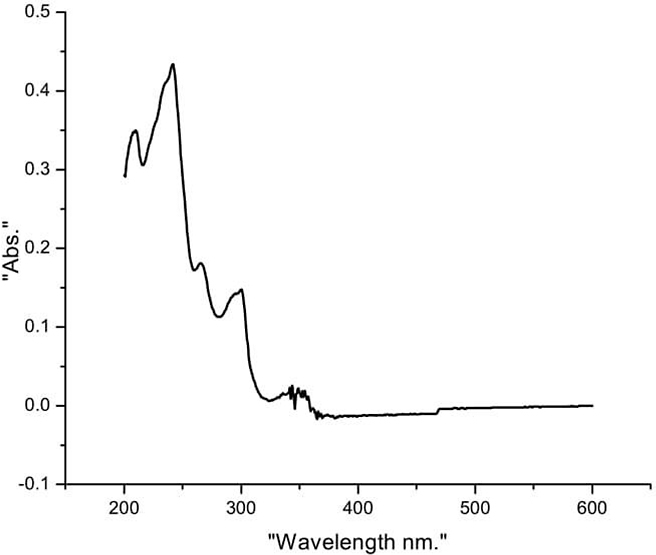
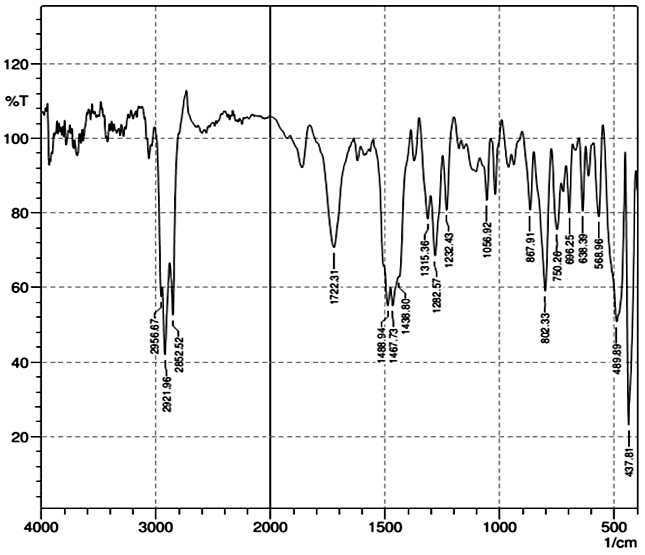
Results & Discussion The compound synthesized in every step was subjected to the purification process. The purity of the compound is checked by TLC. The synthesized compounds were characterized by UV-Visible, FT- IR, NMR, and Mass spectroscopic techniques. Characterization of 3, 6 – dibromocarbazole (A) UV-Visible spectra (Ethanol, nm): 363, 353, 338, 303, 267. FT- IR Spectra (KBr, cm-1): 3406, 3068, 1471, 1284, 570 FT-IR gave characteristic peaks 3406 cm-1 indicated NH stretching frequency, 3068 cm-1 indicated the Ar C-H stretching, 1471 cm-1 indicated the Ar C=C stretching, 1284 cm-1 indicated the C-N stretching and 570 cm-1 indicated the C-Br stretching. Characterization of 3, 6 – diethoxycarbazole (B) UV-Visible spectra (Ethanol, nm): 350, 336, 303, 266 In UV-Visible spectra, the λmax of 3,6-Diethoxy carbazole is observed at 350 nm. FT- IR Spectra (KBr, cm-1): 3409, 1326, 1053 FT-IR gave characteristic peaks at 3409 indicated NH stretching frequency, 1326 cm-1 indicated the Ar C=C stretching and the peak at 1053 cm-1 is due to C-O stretch. Characterization of tris(4-bromophenyl)amine (C) UV-Visible spectra (Ethanol, nm): 315, 290 FT- IR Spectra (KBr, cm-1): 3068, 2950, 740 The peak at 3068 cm-1 and 2950 cm-1 indicated aromatic -C-H stretch, the peak at 740 cm-1 indicated C-Br stretch Characterization of tris(4-(3,6-diethoxy-9H-Carbazol-9 yl)phenyl)amine (D) UV-Visible spectra (Chloroform, nm): 354, 301, 250 In UV-Visible spectra, the λmax of the coupling product is observed at 354 nm. This may indicate that the coupling reaction makes the compound to bathochromic shift when comparing to the individual compounds.
FT- IR Spectra (KBr, cm-1): 3070, 1326. FT-IR gave characteristic peaks 3070 cm-1 indicated the Ar C-H stretching, 1326 cm-1 indicated the Ar C=C stretching. The peaks due to N-H stretch and C-Br stretch are absent, this confirms the formation of the coupling product.
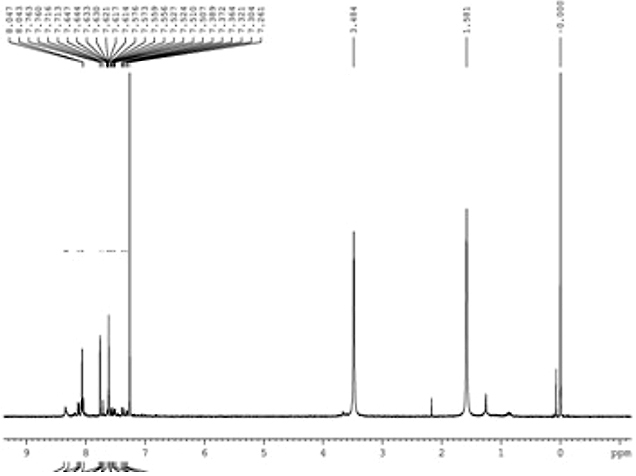
1H NMR Spectra (CDCl3, ppm): 7.1-7.9 (m, 30 H), 3.48 (s, 12H), 1.5 (s, 18 H) The NMR spectrum confirms the presence of aromatic ring at 7.1-7.9δ, –OCH2 group at 3.48δ and –CH3 group at 1.5δ.
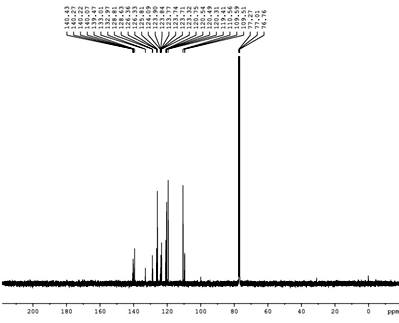
13C-NMR spectra (CDCl3, ppm): 109,119,120,123,125,128,132,140 (Ar-C), 77 (-OCH2) In 13C-NMR spectrum aromatic carbons at 109 ppm-140 ppm and ethoxy carbon at 77 ppm.
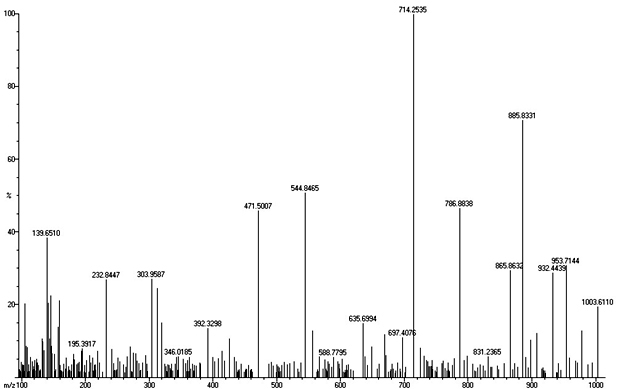
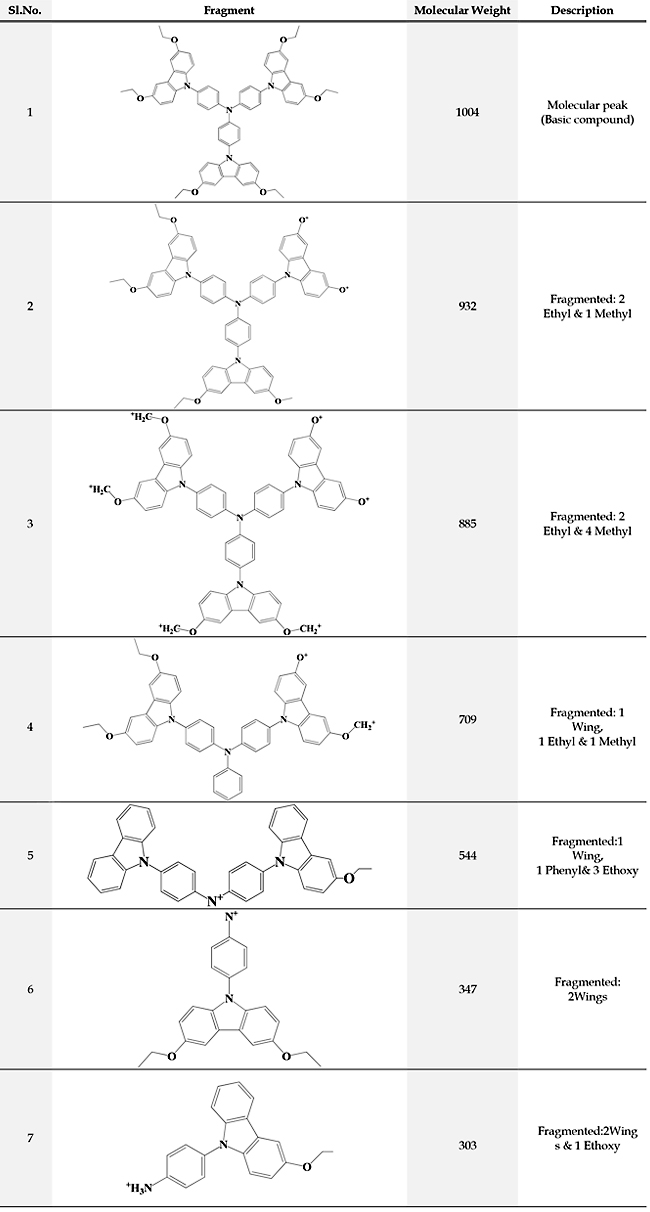
Mass Spectra: m/z 1004(M+)
The structure of the synthesized compound tris(4-(3,6-diethoxy-9H-Carbazol-9 yl) phenyl)amine is confirmed from the spectral data. Conclusion The compound tris(4-(3,6-diethoxy-9H-Carbazol-9 yl)phenyl)amine was synthesized by Ullmann coupling. Various intermediate compounds were synthesized by multistep organic reactions. The synthesized compounds were characterized by UV-Visible, FT-IR, NMR, and Mass spectroscopic techniques. The spectral data confirm the structure of the compound. The synthesized compound is expected to possess a good hole transporting property. Acknowledgment One of the authors (C. Saritha) acknowledges the financial support from Kerala State Council for Science Technology and Environment (KSCSTE) –Women Scientist Division. References
|