|
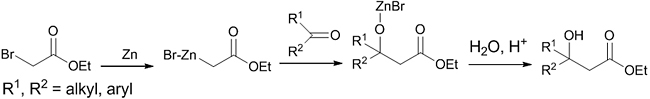
Introduction The classical Reformatsky reaction was discovered by Sergei Nikolayevich Reformatsky in 1887 [1]. It consists of a zinc-induced reaction between ∝-haloesters and aldehyde or ketone to produce β-hydroxy esters (Scheme 1), which are valuable precursors in natural product synthesis and pharmaceuticals. Generally, in this reaction, the enolate is formed by the oxidative addition of a metal or low-valent metal salt or complex into a carbon-halogen bond, activated by a vicinal carbonyl-derived group, followed by a reaction between enolate and appropriate electrophile [2]. It was reported that other metals like Sm, Cr, Sn, Ti, Co, In or Fe in low oxidation states are also suited for this reaction [3]. An aqueous metal-free electrochemical Reformatsky reaction is also known [4]. The solvents used are generally ethers such as Et2O, THF, and 1,4-dioxane. The most important application is that this reaction takes place in neutral conditions, whereas the aldol reaction is a base or acid catalyzed reaction. The disadvantages are lower yield and stereoselectivity. Much progress has been made in this field to enhance the stereoselectivity by using chiral ligands. Furstner et al. introduced the catalytic redox cycle of this reaction [5]. Cozzi highlighted the possibility of catalytic enantioselective and diastereoselective Reformatsky reaction with different electrophiles [6]. A recent review describes the highly diastereoselective Reformatsky reactions using various chiral auxiliaries, either on the electrophile or on the nucleophile, in presence of different metals, which gave rise to high yield products [7]. In the same year, another review on highly enantioselective asymmetric Reformatsky reaction using chiral catalysts was also published [8]. The first example of an enantioselective Reformatsky reaction was reported in 1973 by using (-)-sparteine as the reagent [9]. In 1991, Soai reported an enantioselective Reformatsky reaction with the use of a chiral amino alcohol ligand (Table 1, entry 1), gave rise to the product with 91% yield and 75% enantiomeric excess (ee) [10]. This aroused great interest in the minds of organic chemists and led to the development of different chiral ligands for asymmetric Reformatsky reaction (Table1) [11].
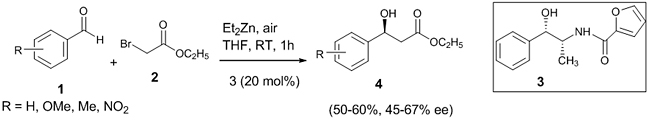
Enantioselective Reformatsky Reaction Enantioselective Reformatsky reactions have been developed using chiral ligands for the formation of new C-C bonds. Asymmetric Reformatsky reaction involving aldehyde A chiral amide ligand 3 was developed from (1S, 2R)-(+)-norephedrine and 2-furoic acid and applied in Reformatsky reaction which enhanced the enantioselectivity of the product. The asymmetric Reformatsky reaction was carried out between various substituted benzaldehydes 1 and α-bromomethyl acetate 2 in presence of chiral ligand 3 (10-30 mol%) mediated by Et2Zn, which gave the corresponding β-hydroxy ester 4 in 50-60% yield and 45-67% ee (enantiomeric excess) (Scheme 2) [19]. Benzaldehydes with electron withdrawing group gave the product with good yield and enantioselectivity.
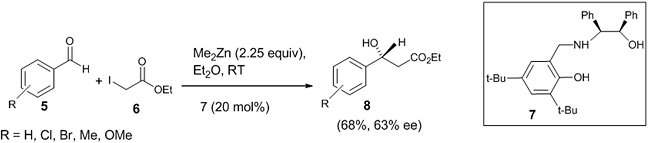
A novel chiral tridentate β-amino alcohols 7 synthesized from a mixture of 2,5-di-tertbutyl salicylaldehyde and chiral (1R, 2S)-2-amino-1,2-diphenylmethanol was used as a catalyst for the asymmetric Reformatsky reaction [20]. Here, the reaction was carried out with variously substituted aldehydes 5 and ethyl iodoacetate 6 to obtain good enantioselectivity of the desired product 8 (Scheme 3). Both electron withdrawing and electron donating groups attached to the benzaldehyde gave enantioselectivity in the range 60-63% ee. It was found that 1-naphthaldehyde and 2-naphthaldehyde gave the best enantioselectivity with 81% and 80% ee, respectively.
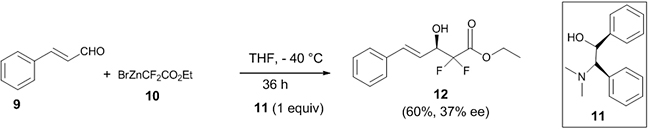
Wu et al. performed an asymmetric Reformatsky reaction between Cinnamaldehyde 9 and ethylbromodifluoroacetate 10 in the presence of the chiral ligand (1S, 2R)-N,N-dimethyl-2-amino-1,2-diphenylmethanol 11 to afford the desired product 12 in 37% ee (Scheme 4) [21].
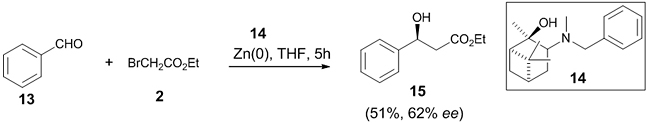
Morita et al. utilized the tertiary amino alcohol 14 obtained from α-pinene as a ligand for the asymmetric Reformatsky reaction between benzaldehyde 13 and 2-bromoethylacetate 2, which yielded the corresponding β-hydroxy ester 15 in 51% yield and 62% ee (Scheme 5) [22].
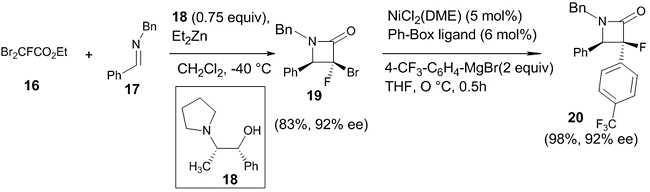
Asymmetric Imino-Reformatsky Reaction The incorporation of fluorine atoms to β-lactams enhanced its antibiotic activity. In 2014, Ando et. al. reported the asymmetric Reformatsky reaction of dibromofluoroacetate 16 with imine 17 using stoichiometric amount of ligand 18 (1R,2S)1-phenyl-2-(1-pyrrolidinyl) propan-1-ol (0.75 equiv) in Et2Zn (3.5 equiv.) at -40° C producing α-bromo-α-fluoro β-lactam 19 with 83% yield and 92% ee (Scheme 6) [23]. The synthetic utility of the reaction was demonstrated by the aryl functionalization of 19 to α-aryl-α-fluoro-β-lactam 20 without any reduction in the optical purity.
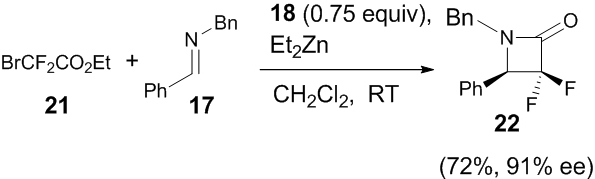
In the same year, Ando and co-workers also investigated a similar synthetic approach for the formation of α,α-difluoro-β-lactam from bromodifluoroacetate 21 with the same imine 17 and catalyst 18 at room temperature, which gave 22 in moderate yield (72%) and good enantioselectivity (91% ee) (Scheme 7) [24]. Imines which contain an electron withdrawing group as substituent in the aromatic ring, gave the corresponding products with high enantioselectivity in the range (94-99% ee) and moderate yield (45-74%), whereas electron donating group exhibited lower enantioselectivity (86-90% ee).
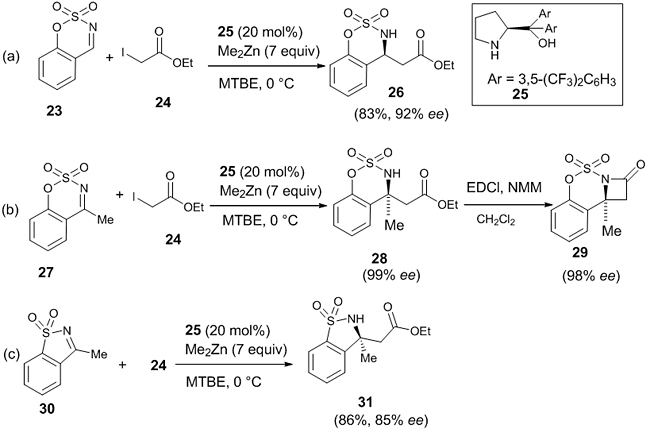
In 2016, De Munck et al. reported the first enantioselective aza-Reformatsky reaction of ethyliodoacetate with cyclic imines (aldimines and ketimines) forming chiral β-amino esters, which are valuable precursors for β-lactams, one of the most important classes of antibiotics [25]. Here, the reaction between cyclic benzo[e][1,2,3]-oxathiazine-2,2-dioxide 23 and ethyliodoacetate 24 in the presence of ligand, (S)-α,α-bis[3,5-bis(trifluoromethyl)phenyl]-2-pyrrolidinemethanol 25 (20 mol%) mediated by Me2Zn afforded the product β-amino ester 26 in good yield (83%) and enantioselectivity (92%) [Scheme 8(a)]. When the amount of ligand was reduced to 10 mol%, the yield and enantioselectivity were reduced to 89% and 87%, respectively. Both electrons withdrawing and electron donating groups attached to the 6-position of the phenyl ring of the cyclic imine gave rise to high enantioselectivity (85-92% ee) and good yield (70-95%). Naphthyl ring substituted to the phenyl ring of cyclic imine gave excellent enantioselectivity (90-93% ee).
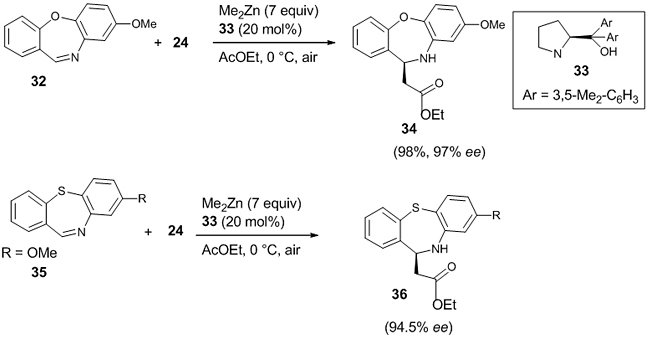
They also reported the enantioselective aza-Reformatsky reaction between cyclic ketimines like 4-methyl-benzo[e][1,2,3] oxathiazine-2,2-dioxide 27 and ethyliodoacetate 24 with the same ligand 25 and reaction condition as in aldimines [Scheme 8(b)]. The chiral β-amino ester 28 thus formed bears a quaternary stereocentre with excellent enantioselectivity (99% ee). Even when the amount of the ligand was reduced to 10 mol%, the enantioselectivity was 97% ee. The chiral β-amino ester can be used for the synthesis of β-lactam 29 without any reduction in the optical purity (98% ee). The reaction was extended successfully in the synthesis of chiral β-amino ester 31 (86%, 85% ee) from the five-membered cyclic N-sulfonyl ketimine 30 under the same reaction conditions [Scheme 8(c)]. Very recently, De Munck et al. published the enantioselective Reformatsky reaction between seven-membered cyclic imine like dibenzo [b, f] [1,4] oxazepine 32 and ethyliodoacetate 24 in the presence of ligand 33 (20 mol %) in Me2Zn and air, and the corresponding β-amino ester 34 was obtained in 98% yield and 97% ee [26]. They also conducted similar Reformatsky reaction between dibenzo[b,f][1,4] thiazepine 35 and 24, affording β-amino ester 36 in 95% yield and 94.5% ee (Scheme 9).
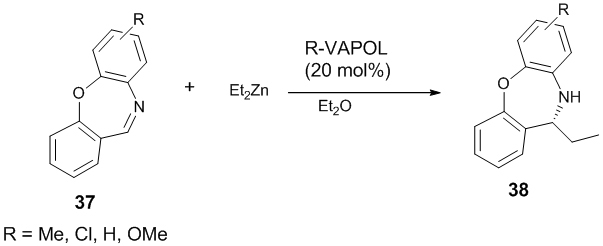
Later on, De Munck et al. extended their work and disclosed the first enantioselective addition of Et2Zn to cyclic imines 37 in presence of (R)-VAPOL-Zn (II) complex as the catalyst [27]. The product 38 was obtained with moderate enantioselectivity (up to 70% ee) and good yield (up to 76%) (Scheme 10).
Diastereoselective Reformatsky Reaction Diastereoselective Reformatsky reactions are widely used to perform the synthesis of cyclic and acyclic moieties using a large variety of chiral auxiliaries which control the stereochemistry of the desired products. Reformatsky reaction on chiral imines Reformatsky reaction between α-oxygenated sulfinylimine 39 and ethyl bromodifluoroacetate 40 has been reported using Honda-Reformatsky condition which afforded the desired product 41 with excellent diastereoselectivity (>94:6) (Scheme 11) [28]. The stereo induction of the product was determined by the configuration of the chiral auxiliary.
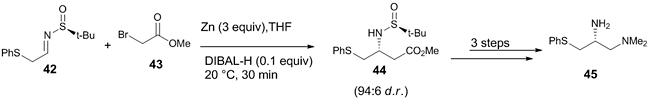
Laclef et al. reported a diastereoselective aza-Reformatsky reaction for the synthesis of the key fragment diamine present in anti-apoptotic Bcl-2/Bcl-XL protein inhibitors, used in anticancer therapy [29]. They performed a large-scale synthesis of the diamine with fewer purification processes. The Reformatsky reaction between sulfinimine 42 and excess of methylbromoacetate 43, afforded the corresponding product 44 with a high diastereomeric ratio (>94:6) which was further transformed to give the diamine fragment 45 with 95% HPLC purity (Scheme 12).
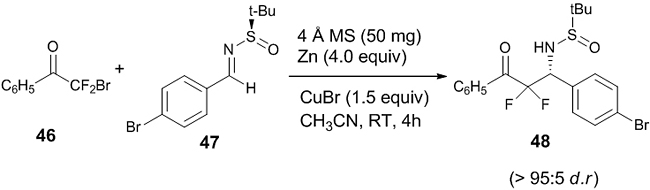
An asymmetric Reformatsky reaction between bromodifluoromethyl phenyl ketone 46 and various substituted chiral ketimines 47 in presence of 4 Å molecular sieves (MS) was reported (Scheme 13) [30]. Both electron withdrawing and electron donating groups on the chiral ketimine afforded the product 48 with excellent diastereoselectivity (>95:5 d.r.) which has many synthetic applications.
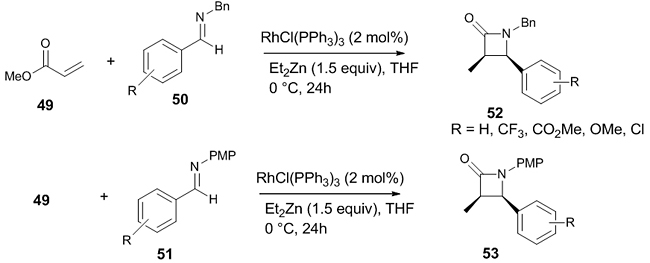
Rhodium enolate based Reformatsky reaction in presence of Wilkinson catalyst and Zn has also been developed [31]. Here, the reaction was conducted on α,β-unsaturated esters 49 and various imines like N-benzyl substituted imines 50 and N-para-methoxy phenyl imines 51 resulting in syn β-lactam 52 and 53 as the products (Scheme 14). Both electron withdrawing and electron donating groups in the benzene ring produced the syn β-lactams in better yields. This method can be used for the one-pot synthesis of spiro β-lactams from ketimines.
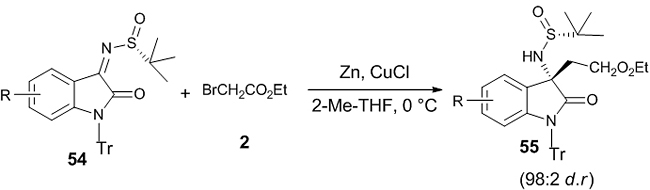
A highly efficient diastereoselective asymmetric Reformatsky reaction between isatin-derived chiral N-sulfinyl ketimines 54 and ethyl bromoacetate 2 in presence of the solvent 2-methyltetrahydrofuran and mediated by Zn-CuCl has been developed in which optically pure 2-oxoindolinyl β3,3-amino acid ester 55 was obtained with excellent diastereoselectivity (98:2 d.r) (Scheme 15) [32]. The reaction was performed with different ketimines and concluded that both electrons withdrawing and electron donating groups in the benzene ring of the ketimine gave rise to better yield and excellent diastereoselectivity. The β-amino acid so obtained was used for the synthesis of gastrin/cholecytokinin-B receptor antagonist AG-041R.
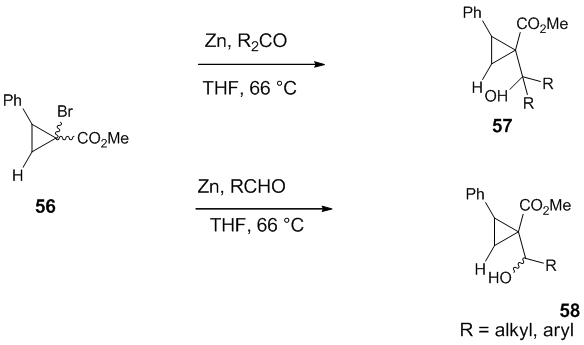
Reformatsky reaction involving chiral auxiliary on cyclopropane ring Nishii et al. developed a Reformatsky reaction between α-bromocyclopropanecarboxylate 56 and ketones, in presence of zinc affording trans adducts 57 (99:1 d.r) in high yield [33]. When the same reaction was carried out in presence of aldehydes, the product 58 was obtained with excellent trans selectivity at the α-position (99:1 d.r) and moderate diastereoselectivity at the β-position (62:38 d.r) (Scheme 16).
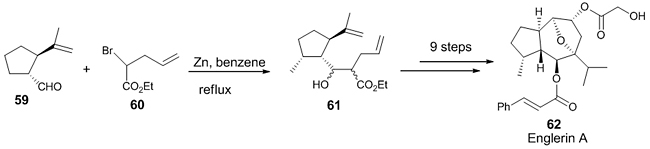
Applications of Reformatsky Reaction in the Total Synthesis of Natural Products Reformatsky reaction has been applied in a large number of total synthesis of natural products. We have categorized the reactions based on the metal complex used. Zinc-mediated Reformatsky reaction Different Zn complexes will show different reactivities, for example, the weak nucleophilic Me2Zn was found to be less reactive than Et2Zn [34]. Zinc reacts slowly with halo compound due to the formation of the oxide coating on Zinc. This problem was solved to some extent by using activated Zn like Rieke-Zn [35] or Cu-Zn alloy [36]. Later, Knochel developed a practical method for the activation of Zn. It was reported that for the synthesis of β-hydroxyesters, ZnCl2 or SnCl2 reduced with sodium in liquid ammonia was used. Some additives in combination with zinc are also used for this reaction which includes tantalum, vanadium binuclear complex, Hg2Cl2, triethyl boron, cerium (III) salts, etc. A short synthetic route for the natural product, (-)-Englerin A (active against renal cancer cell lines) 62 was reported with 12 steps in 16% yield [37]. The first step in the construction of the hydroazulene framework was a zinc-mediated reaction between photocitral A 59 and bromoester 60 under mild conditions, affording the product 61 in good yield as a diastereomeric mixture (Scheme 17).
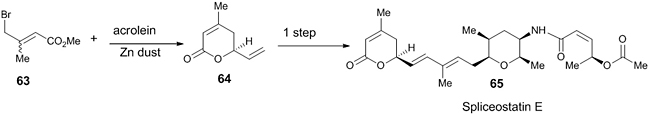
Ghosh et al. performed zinc-mediated reaction for the formation of racemic dihydropyranone from methyl-3-bromomethylcrotonate 63 and acrolein as one of the steps in the synthesis of Spliceostatin E 65, which has potent antitumor activity (Scheme 18) [38]. The racemic mixture was resolved by chiral HPLC and the (S)-dihydropyranone 64 was used for the synthesis.
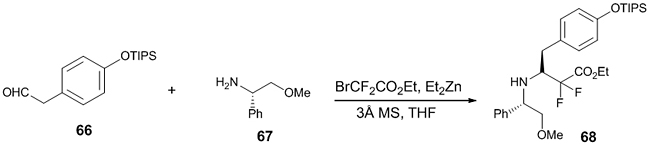
The synthesis of Tyr1-Ψ [(Z) CF=CH]-Gly2 Leu-enkephalin fluorinated peptidomimetics have been reported using Reformatsky reaction [39]. In the key step, fluorine was incorporated through a diastereoselective Reformatsky-Honda reaction between TIPS protected phenyl acetaldehyde 66 and chiral amine 67 using molecular sieves, and the desired product 68 was obtained in good yield (Scheme 19).
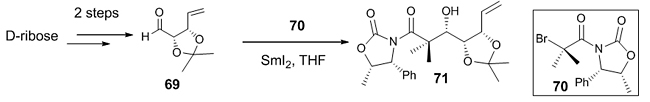
Samarium-mediated Reformatsky reaction High diastereoselective products can be obtained by using a one-electron reducing agent, Samarium diiodide, due to its moderate oxidation potential and high oxophilicity. Samarium-mediated Reformatsky reaction proceeds through an intramolecular reaction yielding medium to largely sized carbocycles [1(b)]. Metallic Samarium can be used in combination with Cadmium (II) chloride or Bismuth (III) Chloride to produce β-hydroxy ketones in moderate to good yields from α-bromoacetophenone and aldehydes in THF-H2O. The key step for the synthesis of the eastern fragment of PI-3- a jatrophane diterpene used in the treatment of cancerous conditions, swellings and warts was a diastereoselective SmI2-mediated Reformatsky reaction [40]. Here, D-ribose was converted to an unsaturated aldehyde 69 which was further reacted with bromoacyl oxazolidinone 70 to afford the oxazolidinone product 71 as a single diastereomer with S-configured hydroxyl group (Scheme 20).
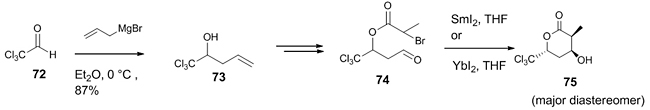
The importance of substituted lactones in the synthesis of natural products encouraged Schulze et al. to synthesize substituted valerolactones by the intramolecular SmI2/YI2-mediated Reformatsky reaction [41]. The synthesis involved the preparation of intermediate 73 from allyl magnesium bromide and chloral 72, which was further transformed into the ester 74 and underwent an intramolecular Reformatsky reaction to produce four diastereomeric products, out of which the major product 75 was the 4,6-anti-configured diastereomer as determined by NMR (Scheme 21).
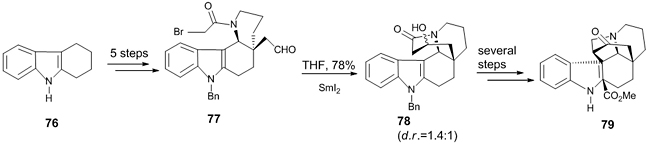
An indole alkaloid, Methyl N-decarbomethoxychanofruticosinate 79, used in the treatment of pharyngitis, tonsillitis, rheumatoid arthritis, etc., has been synthesized using Reformatsky reaction [42]. The commercially available 1, 2, 3, 4-tetrahydro-4-oxocarbazole 76 was transformed to aldehyde 77. In order to create a seven-membered ring, an intramolecular SmI2-mediated Reformatsky reaction was performed to give 78, which underwent several steps to afford the product as a mixture of two diastereomers in good yield (Scheme 22).
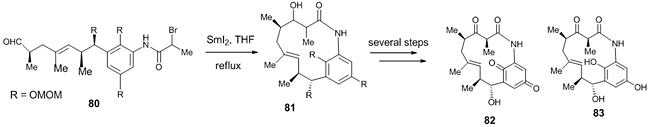
One of the key steps in the total synthesis of Ansamacrolactams (+)-Q-1047H-A-A 82 and (+)-Q-1047H-R-A 83 (class of macrolactam bacterial metabolites, used for the synthesis of some natural products) was the SmI2-mediated intramolecular Reformatsky reaction for the formation of macrolactam ring 81 from the intermediate 80 (Scheme 23) [43].
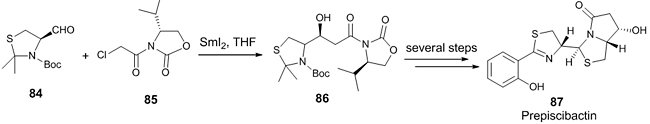
The first total synthesis of Prepiscibactin 87, the key intermediate for the biosynthesis of piscibactin (siderophore seen in bacterium Photobacterium damselae) was realized by SmI2-mediated Reformatsky reaction [44]. The key step in the synthesis was a SmI2-mediated Reformatsky reaction between thiazolidinic aldehyde 84 and α-chloroacetyl-2-oxazolidinone 85 affording the syn-selective product 86 with 99% d.r. (Scheme 24).
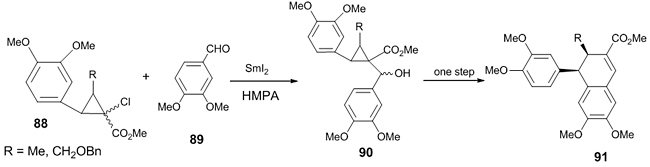
Nishii et al. reported the total synthesis of Cyclogalgravin and its dicarboxyl analog due to its biological importance such as antineoplastic cytotoxicity and apoptosis-inducing activities [45]. One of the key steps in this synthesis was a SmI2-mediated Reformatsky reaction between α-chloroester 88 and veratraldehyde 89. An excellent trans selective product 90 (trans/cis = 99/1) was obtained in good yields which on ring expansion gave Cyclogalgravin 91 (Scheme 25).
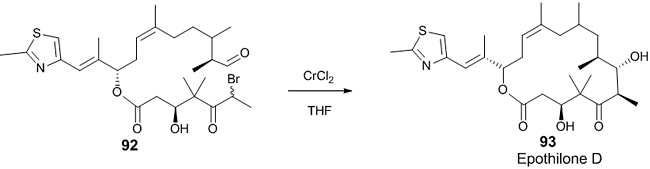
Chromium-mediated Reformatsky reaction Chromium is rarely used in the Reformatsky reaction. A chromium-mediated Reformatsky reaction is a powerful tool for the formation of the asymmetric C-C bond due to its unusual stereospecificity and chemoselectivity in macroaldolization. In this reaction, when aldehydes are used as electrophiles, diastereoselective syn products are obtained whereas anti products are obtained with chiral auxiliaries. A chromium-mediated Reformatsky reaction was used in the total synthesis of Epothilone D 93 [46]. The required syn selective C6-C7 bond of this compound was prepared from the precursor 92 in presence of CrCl2. (Scheme 26).
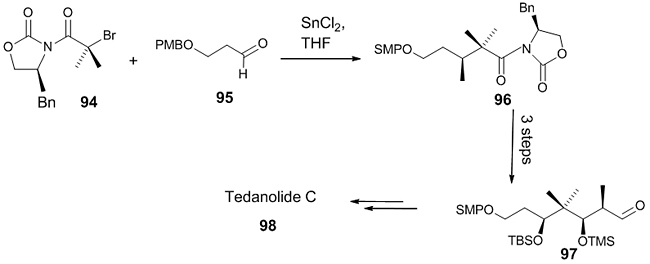
Tin-mediated Reformatsky reaction Tedanolide C 98 is a marine natural product isolated from a Papua New Guinea sponge which showed excellent potency towards colorectal cancer cell line and is also a protein synthesis inhibitor. The starting material 97 for the synthesis of a C1-C11 fragment of Tedanolide C was synthesized from oxazolidinone 94 with an aldehyde 95, by a tin-mediated Reformatsky reaction which gave rise to a product 96 with 56% yield and >98:2 d.r. (Scheme 27) [47].
Conclusions Asymmetric Reformatsky reaction facilitates the formation of a C-C bond between a halide and an aldehyde which leads to the formation of a chiral alcohol. This review covers the asymmetric Reformatsky reaction using different transition metals and various chiral ligands. Both enantioselective and diastereoselective Reformatsky reactions are discussed. In addition to Zinc, other transition metals such as Sm-, Sn-, and Cr-mediated Reformatsky reactions have also evolved, affording the products in excellent yields due to their improved reactivity. The incorporation of fluorine into β-lactam has become a major research interest due to the enhanced antibiotic activity. Much progress has been observed in the intramolecular Reformatsky reaction using SmI2, which was also included in this review. References
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||