|
Introduction Breast cancer is a frequent malignant tumor that kills women, and its prevalence rate in Iraq is growing year after year. The fatality rate from breast cancer is declining, despite the disease's higher incidence rate, due to early detection and improved treatment [1]. Serum tumor indicators are useful in early diagnosis, detecting disease progression, recurrence, tumor metastasis, and evaluating therapy effectiveness [2]. The cancer antigen CA15.3 is a mucin that belongs to a wide family of glycoproteins released by breast cancer cells, and its levels may rise as the disease advances and fall when the tumor reacts to cancer therapy [3]. CA15.3 levels may be greater than normal in cancers of the lung, pancreas, ovary, and prostate, but not as high as in breast cancer. Endometriosis, pelvic inflammatory disease and liver illness are examples of non-cancerous diseases that raise CA15.3. It is also possible to rise during pregnancy [4].Carcinoembryonic antigen (CEA) is a protein that has a role in cell adhesion. CEA is generally created throughout fetal development; however, it is no longer produced prior to delivery. As a result, it is seldom seen in the blood of healthy people [5]. CEA levels can be found to be elevated in cancers of the colon, lung, liver, breast, prostate, pancreas, ovary, and stomach. It is also elevated in several benign conditions, including inflammatory bowel disease, Crohn's disease, pulmonary infection, and renal failure. Levels are also increased in smokers [6]. Alkaline phosphatase is a member of the hydrolysis enzyme family. ALP is mostly derived from the bones and liver in healthy persons, with modest contributions from the kidney and leukocytes [8]. In most cases, a high serum ALP content is connected with bile obstruction, cholestasis, liver illness, hepatitis, and malignancy. People with primary and metastatic liver and bone cancers, such as colorectal cancer hepatic malignancies and breast cancer with bone and liver involvement, have higher serum ALP levels [9]. Alkaline phosphatase determination (ALP) isoenzyme activity can aid in the diagnosis and clinical assessment of cancer patients.
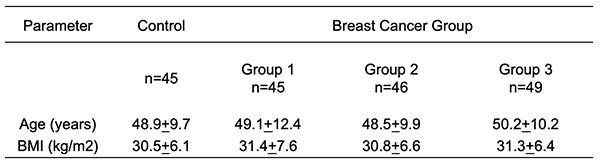
Materials and Methods Patients: Serum samples were collected from 185 women aged 25 to 65 years, 45 of these women were control and 140 women were breast cancer patients attending the oncology and nuclear medicine hospital in Mosul from February 2020 to March 2021. Group 1: 45 patients with (stages I and II), 46 individuals with (stage III), and 49 patients with (stage IV). All the patients had just been diagnosed with breast cancer and had had no treatment, radiation, chemotherapy, or any other type of hormone therapy. Obtaining Blood Samples: All patients and controls had 5 mL of blood drawn from them, and the serum was centrifuged for 15 minutes at 4000 rpm before being stored at -20°C until analysis. Methods: CA15.3, CEA and vitamin D3 levels in serum were determined by using an Enzyme Immunoassay kit based on the principle of Enzyme-Linked Immunosorbent Assay [ELSA] (Dinabot, Tokyo, Japan). Serum calcium, alkaline phosphatase, uric acid, creatinine, and urea were measured by colorimetric method using a kit of Biolabo's manufacturing. A colorimetric approach was performed for the determination of serum calcium according to procedure in Panteghini et al. (2012) [10], alkaline phosphatase activity was estimated according to procedure in Kind and King (1954) [11], and serum uric acid was determined by an enzymatic method according to procedure in Burtis et al. (2015) [12]. Serum creatinine was estimated using the colorimetric method according to procedure in Mažeikienė and Kaminskas (2012) [13] and urea serum was estimated by the same method according to procedure in Burtis et al. (1999) [14].Statistical analysis: SPSS 17 was used to compute the mean and standard deviation for all statistics in the study (SD). The T-Test was used to examine the importance of the distinction in mean values. A p 0.05 number indicates significant, whereas a p >0.05 value indicates non-significant.
Results & Discussion The following are the results of the several biochemical parameters examined in the study for patients and controls:
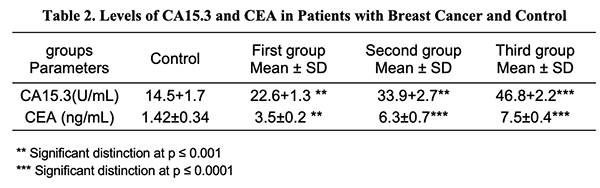
CA15.3 in Breast Serum Cancer: The data in Table 2 revealed a substantial rise (p 0.001) in serum CA15.3 in the first and second groups, and a significant increase (p 0.0001) in serum CA15.3 in the third group of breast cancer patients vs. control groupings. The results are congruent with those of prior studies which have shown that tumor markers CA15.3 are greater in advanced case breast cancer than in early-stage breast cancer [15]. Additionally, a recent study by Khushk et al. (2021) [16] showed that patients with malignant tumors had considerably higher CEA and CA15.3 values than a person with type I breast cancer [17], underlining the significance of serum CA15.3 as a useful marker for tracking the course of breast cancer and detecting metastasis in patients [18].
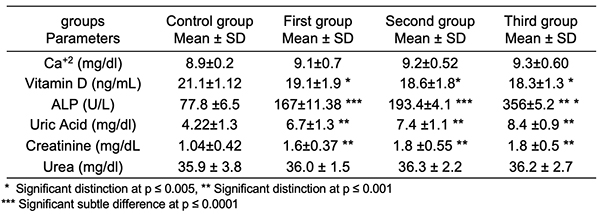
Serum CEA in Breast Cancer: The results in Table 2 demonstrated that there was a considerable rise (p < 0.001) in concentrations in the blood serum of CEA in the first group of cancer patients, and a significant rise (p < 0.0001) in CEA serum in the second and third groups compared to breast cancer patients within the control groups. A similar finding has been reported by Mohammed et al. (2021) which showed that increased serum levels of CEA were shown in stage III breast cancer [19] and consistent with the study by Yerushalmi et al. (2012) which showed a significant correlation between elevated serum tumor marker levels and tumor stage [20]. Consequently, the usefulness of serum markers for cancer detection is stressed [21]. Serum Calcium in Breast Cancer: The results in Table 3 show that the serum calcium concentrations of women with breast cancer did not differ significantly (p > 0.05). no significant subtle difference at (p > 0.05) in serum calcium in the groups of breast cancer women. These findings concur with earlier research [22,23] that found there is no proven connection between the stage of a tumor and calcium levels and subsequent research discovered no link between overall calcium and risk of cancer in postmenopausal individuals [24]. It is generally documented that calcium functions as an intracellular messenger in cell proliferation, death, as well as the transmission of a wide variety of signals [25].
Serum Vitamin D3 in Breast Cancer: The results in Table 3 showed a considerable reduction (p > 0.05) in serum D3 vitamin levels in all groups of cancer breast patients. Vitamin D levels and the risk of developing breast cancer have been proven to be inversely related in numerous studies [26,27]. Another study by Park et al. (2015) discovered that blood vitamin D levels of 20 ng/mL were related with a 27% increased risk of breast cancer than someone with appropriate vitamin D levels (25(OH)D > 20 ng/ml) [28]. According to a study by Mawer et al. (1997), blood levels of 25(OH)D3 decrease with increasing tumor stage in breast cancer [29] and that vitamin D3's active form, 1,25(OH)2D, has an anticancer impact by promoting cellular differentiation, activating apoptosis, blocking angiogenesis, and limiting cancer cell development [30]. Vitamin D has a vital role in inducing apoptosis, neuronal differentiation promotion, has anti-inflammatory and antiangiogenic effects, and suppresses angiogenesis, invasion and metastasis [31]. Serum Alkaline Phosphatase in Breast Cancer: Clearly, as seen in Table 3, there was a sharp rise (p ≤0.0001) in alkaline phosphatase serum (ALP) in all groups of breast cancer patients all groups of as compared to the control. These findings support prior research that found a considerable increase in non-metastatic cases, with the number of cases without metastasis going up by a factor of six, while the number of cases with metastasis went up by a factor of four. [32]. Similar to Singh et al. (2013), who discovered a considerable increase in ALP levels at various carcinogenesis development stages [33], Mishra et al. (2004) discovered a continuous increase in ALP levels in metastasis [34]. The increase in ALP indicates that the malignancy has progressed to the bones or liver [35]. The increased serum ALP is caused by the enzyme's quicker de novo synthesis and subsequent regurgitation into the serum. In breast cancer patients, a gradual rise in serum ALP activity is a sign of metastasis [34]. Serum in Uric Acid Breast Cancer: The outcomes listed in Table 2 revealed a considerable rise (p ≤0.001) in serum uric acid in all groups of breast cancer women as compared to the control. A similar finding has been reported by other investigators [36]. A high amount of serum uric acid is linked to a number of illnesses, the most common of which is renal failure. According to Veni et al. (2011), the dramatically increased uric acid levels in women with breast cancer who have not received any treatment may be connected to oxidative stress [37]. Serum Creatinine Breast Cancer: Tables 2 and 3 demonstrate that serum creatinine levels in the first, second, and third groups of breast cancer women rise significantly (p ≤ 0.001) in contrast to healthy controls. Creatinine levels in the blood are regarded as more responsive than BUN in determining renal function. Because renal illness is the sole reason for elevated creatinine levels, Devi et al. observed an increase in creatinine levels in 2015 [38]. Serum Urea in Breast Cancer: The statistical examination of the data revealed no significant change in serum urea between these groups (p >0.05). All of the groups' results were within the normal range, which is consistent with previous findings [38].
Conclusion Patients with advanced forms of breast cancer reported considerably higher levels of CEA and CA15.3 than those with early-type breast cancer, implying that these tumor markers' serum levels may be more efficient than early detection in maintaining advanced malignancies and may have a vital role in the early detection of metastasis in breast cancer patients. The examination of serum biochemical characteristics might be a useful diagnostic tool for illness and metastatic surveillance.
References
|