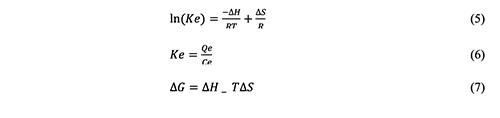|
Introduction Environmental pollution is one of the main issues people nowadays have to deal with, and it becomes more dangerous due to numerous human activities. It has been discovered that environmental pollution is closely related to the global population growth [1]. One of the components that keeps life alive is water. Since millions of chemical compounds are discharged every day, directly or indirectly, to water sources without any treatment [2], freshwater supplies have recently seen a major decline as a result of technological advancement [3]. As a result, the issue of water contamination has attracted a lot of attention in the modern era [1]. Dyes are considered organic pollutants in aqueous systems and can pose a risk to all elements of the environment due to its toxicity, especially when they are present in high concentrations [4]. Dyes include all substances used to color textiles, leather, food, and other materials. Organic compounds are one of the most important components of industrial wastewater. There is a significant risk of long-term consequences since some organic pollutants have the potential to cause malignant diseases [5]. According to WHO reports, the bulk of diseases that spread in undeveloped countries are mostly caused by contaminated drinking water. Researchers have therefore used a variety of techniques to clean industrial water [6,7]. Diverse techniques have been used to cleanse and remove organic pollutants from industrial water. They comprise ion exchange, chemical oxidation, photo-oxidation, and the adsorption process, among others [8]. Adsorption is a method that is both efficient and affordable. Data from the WHO show that it is frequently used to clean up contaminated water [9-12]. Nanotechnology is used by many industries and sectors, such as the chemical industry, photoelectrochemical applications, environmental health, medicine, and energy [13,14]. Nanotechnology [15], a crucial advancement in modern science, has made it possible to produce materials with distinctive size, structure, and substance. Production, processing, and application all involve materials with a diameter less than a nanometer [16,17]. Physical, chemical, as well as biological characteristics at the nanoscale differ from bulk atoms and molecules individually [18-20]. This enables the creation of novel classes of cutting-edge chemicals and materials to satisfy the requirements of high-tech applications [21-24]. Cerium oxide (CeO2), commonly referred to as nano ceria, is a superior semiconducting substance with a broad band gap energy of 3.19 eV, making it a strong choice for catalytic applications [25]. Due to their potential benefits in a number of applications, including a catalyst, an electrolyte material for solid oxide fuel cells, a diesel fuel additive, a material of high refractive index, an insulating layer on silicon substrates, gas sensors, and more recently biomedicine, significant efforts have been made in recent years to develop new synthetic methods for the preparation of nanostructure cerium oxides [26-30]. The biological impacts of nano ceria have been covered in numerous studies. It is well recognized that cerium oxide nanoparticles, in contrast to other metal oxide nanoparticles, do not have cytotoxic effects and instead offer protection against a variety of cellular damages, such as radiological shocks that encourage the generation of free radicals [31,32]. Cerium oxide nanoparticles have been created using a variety of techniques [33]. In this study, the green synthesis approach was used to make cerium oxide nanoparticles and used it to remove the Cibacron red dye, which is one of the dyes used in the textile industry in the Wasit Governorate and most of which is disposed of as wastewater. The synthesis of nanoparticles using plant extracts is a clean and safe method that does not contain harmful chemicals, but rather uses natural materials from plants.
Experimental Extraction of the Watercress Plant Extract Watercress plant extract has been collected and washed with deionized water to get rid of any dust. The dry leaves are gently blended in a mixer to obtain homogeneous powders. Then, 10 grams of leaves were ground and combined with 150 ml of deionized water. The mixture was then heated for 30 minutes at 60°C while being stirred. After filtering, the solution was placed in the refrigerator. Synthesis of CeO2 Nanoparticles Cerium oxide nanoparticles were created using the green synthesis method. Accordingly, 400 ml of watercress filter had added to 0.01 mole of Ce(NO3)3 slowly (one drop every second) and stirred for 30 minutes. The yellow powder was repeatedly rinsed with deionized water, separated, and precipitated. After being dried for an hour at 150°C, the precipitate was calcined for 3 hours at 400°C. The copper oxide nanoparticles were produced as a yellowish white powder. Cibacron Red Dye Adsorption on CeO2 NPs The equilibrium isotherm of a particular adsorbent serves as a representation of its adsorbent properties for the purpose of designing adsorption operations. A stock solution of the Cibacron red dye (50 ppm) was made in deionized water. 0.01 g of CeO2 nanoparticles were added to 10 ml of dye solution, and they were then heated to 298 K for 30 minutes. After the solution had been filtered, the dye concentration in the filtrate was measured using a UV-visible absorption spectrophotometer [34-35].
where Qe (mg/g) is the equilibrium adsorption capacity, C and Ce are the beginning as well as equilibrium concentrations of Cibacron red dye, and M is the mass of the CeO2 nanoparticles (g). V sol is therefore the volume of Cibacron red (L). Cerium Oxide Nanoparticles Characterization X-ray diffraction was used to investigate the sample of CeO2 nanoparticles (XRD-6000). The morphology of nanoparticles was studied using transmission electron microscopy. The shape of the CeO2 nanoparticles was studied under a scanning electron microscope (SEM). Nanoparticle morphology was examined using transmission electron microscopy (TEM).
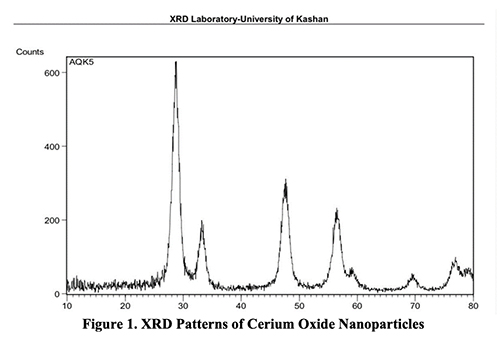
Result and Discussion The X-ray Diffraction of CeO2 Nanoparticles X-ray diffraction (XRD) in the 2θ range of 200-700 (Rigaku Miniflex II) utilizing Cu K radiations (λ = 1.54A) operated at a voltage of 30 kV and current of 15 mA was used to analyze the XRD pattern of cerium oxide nanoparticles displayed in Figure 1. The (111), (200), (220), (311), (222), and (400) cubic phase peaks are the diffracted peaks observed at diffraction angles 2θ of 28.270, 32.780, 47.050, 55.940, 59.070, and 69.090.
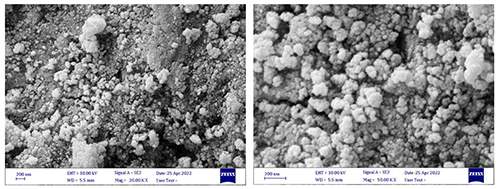
The spectra make it very evident that there are no additional contaminant peaks. Debye-Scherer equation [36] was used to calculate the crystallite size of cerium oxide nanoparticles, which was found to be 22 nm. Field Emission Scanning Electron Microscope of CeO2 Nanoparticles FE-SEM was used to analyze the surface morphology of pure CeO2 nanoparticles that had been calcined at 400°C. The SEM anal- ysis revealed that the prepared sample was formed as spherical aggregates with a reasonably uniform distribution. Figure 2 displays the crystal nature of the equally-sized manufactured nanoparticles.
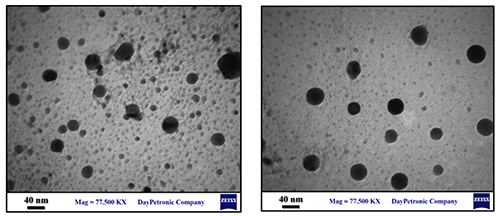
Transmission Electron Microscopy of CeO2 Nanoparticles TEM pictures of the sample were acquired and are displayed in Figure 3 to further the research of the morphology, as well as size, of the product as it was produced. The nanoparticle sizes determined by the XRD diffraction pattern and the TEM picture, which shows average sizes of 36 nm, are closely correlated. Understanding the crystalline characteristics of the nano- particles is the aim of the TEM study.
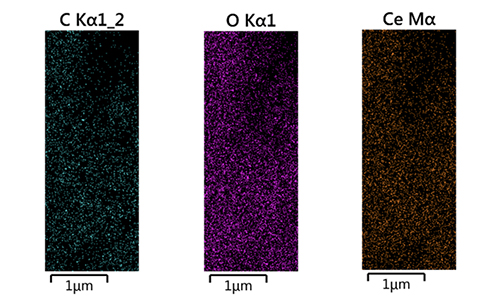
Characterization of Energy-dispersive X-ray Spectroscopes The chemical composition and purity of the as-produced CeO2 nanoparticles were examined using EDS analysis as shown in Figure 4.The presence of Ce, as well as O, in the product is shown by a typical EDX spectrum, as seen in Figure 5. The silicon plates employed in the EDS instrument are what cause the presence of Si peaks in this spectrum.
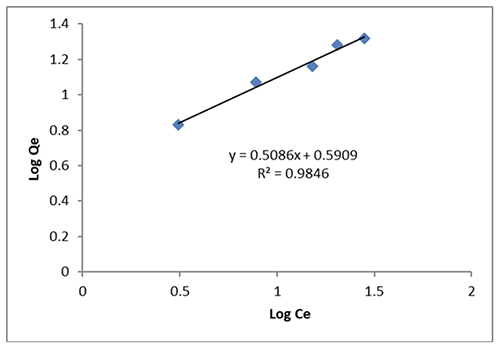
Adsorption Isotherms The major purpose of the adsorption analysis is to evaluate the correlation between the dye and adsorption by contrasting the adsorption isotherm with the adsorption data. This study evaluated both the Lang-muir and Freundlich models. The linear Freundlich adsorption process is represented by the following formula [10] [34-36]:
The Freundlich constants, kf as well as n, respectively, show the adsorption capacity and intensity. As shown in Figure 6, while n is determined using the slope, kf is deter- mined using the intercept. In the current study, 1/n was found to be 0.5086 for the Freundlich CeO2 isotherm. As a result, this research supported the value of physical adsorption. [37].
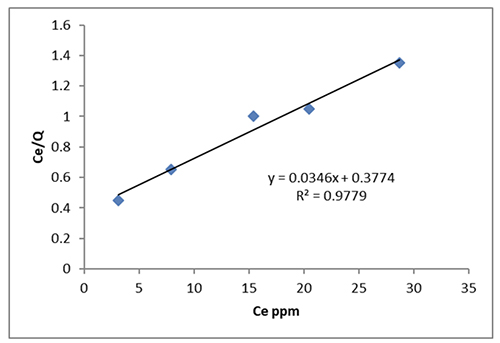
The Freundlich isotherm (R2 = 0.9846) provides a better fit for the adsorption. The equation that follows shows how well the data fits the Langmuir adsorption isotherm [36,37]:
The Langmuir constant is Kl (mg/L), but the maximum adsorption of Cibacron red dye is q max (mg/g). The separation factor, often known as the dimensionless constant (Rl) [36], outlines and illustrates the main features of the Langmuir isotherm:
According to Figure 7, the dye adheres to CeO2 the best when the initial dye concentration is Ci (mg/L) and the Rl values are all between (0-1).
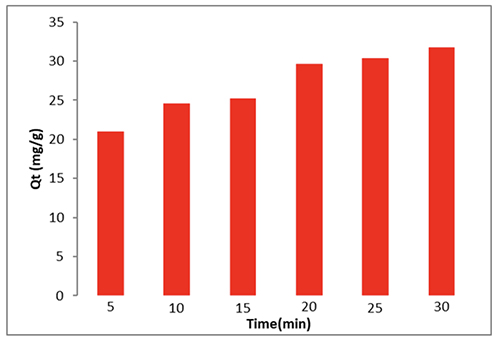
The Effect of Contact Time In series of experiments, 0.01 g CeO2 nanoparticle and also 10 ml (50 ppm) dye were used to determine both contact time as well as equilibrium time. The liquid was warmed to 298 K with the use of a 200 rpm shaker. Adsorption happens rather quickly in the first 5 to 40 minutes. Due to the active CeO2 nanoparticles' close association with the dye, quick adsorption is made feasible. After 35 minutes, the dye adsorption rate stabilizes due to the nanoparticles' surface, as seen in Figure 8.
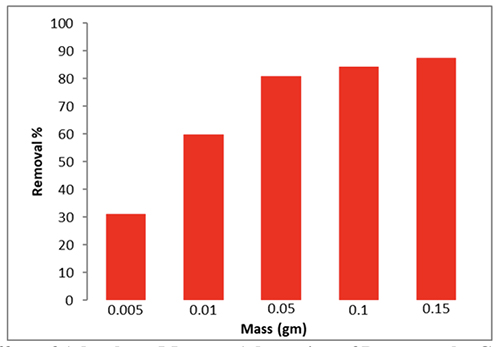
The Effect of Adsorbent Mass Different masses of CeO2 NPs (0.005g, 0.01g, 0.05g, 0.1g, and 0.15g) were added to 50 ppm of dye to evaluate the adsorbent's efficacy. The mixture was shaken at 298 K and 200 rpm. The graph illustrates the link between adsorption volume and mass. First, because nanoparticles have more active sites, adsorption occurs very quickly. Figure 9 shows how increasing the mass of the CeO2 NPs led to an increase in dye ad-sorption.
The Effect of Temperature The effect of temperature on dye adsorption on the surface of CeO2 NPs was investigated at a number of temperatures, including 288 K, 298 K, 308 K, 318 K, and 328 K. With rising temperature, the dye adsorption solution volume increases. As a result, the endothermic process occurs, and the average value of H° increases above zero. This clarifies how the absorption and adsorption processes work. As the temperature increases, the rate of diffusion accelerates, and a strong bond is established with the adsorbent, the diffusion molecules are absorbed by the holes. Since thermodynamic parameters provide exact information on changes in inherent energy brought on by adsorption, thorough evaluation of these parameters is crucial. The following adjustments were examined to estimate the adsorption process utilizing the free energy of adsorption (∆G°), entropy (∆S°), and also enthalpy (∆H°) [36-40]:
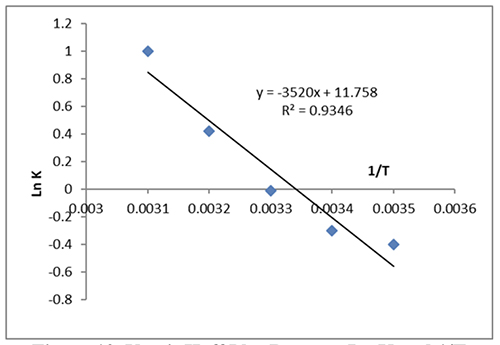
The equilibrium constant, Ke, the gas constant, and the temperature in Kelvin are all equal to 8.314 J/mol K (K). The interaction was endothermic, as shown by the Van 't Hoff plot in Figure 10 between ln K and 1/T, where the ∆H was 29.26 kJ/mole prevented by slope.
The intercept's ∆S, which came out at 97.68 J/mole, showed that the adsorbed particles were still moving very near to the surface. They used the words “absorption” and “adsorption.” The positive ∆G value at 293 K, which is 2.44 KJ/mol, points to non-spontaneous adsorption. Dynamics The kinetics of dye adsorption on the surface adsorbents of CeO2 NPs determines the uses for adsorbents. The dye analysis revealed that the adsorption equilibrium duration for 0.01 g of the CeO2 nanoparticle adsorbents was around 30 minutes. Additionally, the following data regarding adsorption was represented in this study using both classical and kinetic models:
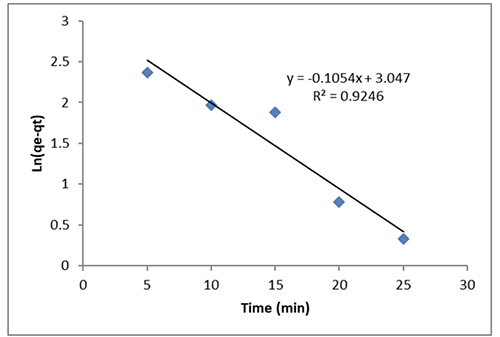
Figure 11 shows the pseudo-first-order rate constant, k1, the equilibrium adsorption capacity, qe (mg g-1), and the amount of dye that has been adsorbed over time, qt (mg g-1) (min-1).
The pseudo-second-order kinetic model is as follows, as seen in [10-12]:
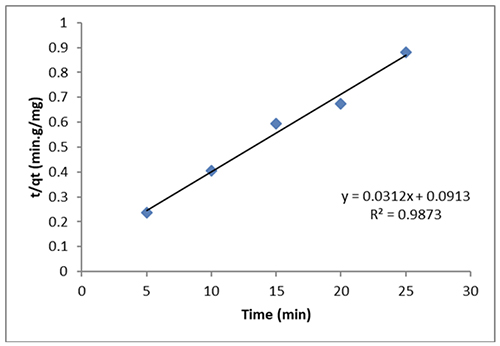
The hypothetical second-order rate constant is denoted by K2. The pseudo-second-order model with a strong association factor (R2 > 0.9873) may adequately capture the kinetic information, as shown in Figure 12.
Conclusion Green synthesis was used to create high-quality CeO2, and XRD, SEM/EDX, and TEM images were taken. The CeO2 NPs' particle sizes varied, according to TEM analysis, and ranged from 28-36 nm. The observed adsorption properties are perfect for removing dye from aqueous solutions. In both kinetic and thermodynamic experiments, CeO2 NPs proved to be effective as adsorbers. The outcomes fit the Langmuir and Freundlich isotherm models rather well. The adsorption is much better captured by the Freundlich isotherm model. The adsorption is endothermic and spontaneous, as per thermodynamics. The slope of the Van 't Hoff plot was used to calculate the enthalpy value, which describes the physical characteristics of the adsorption and is equal to 29.26 kJ/mole. With an R2 value of 0.9873, this adsorption complies with pseudo-second-order
References
|
||||||||||||||||||||||||||||||||||||||||