|
Introduction
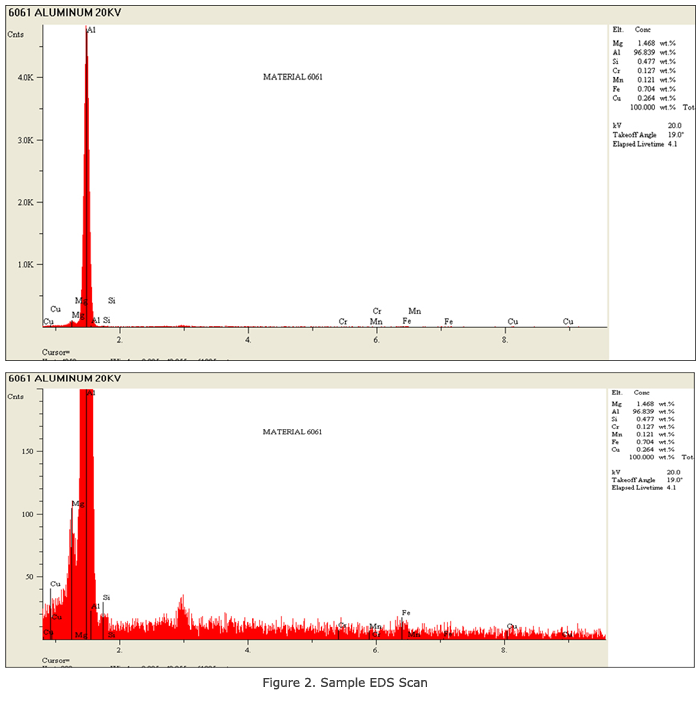
Uses of EDS Analysis on Materials This method is used in many types of analyses. These analyses can include identification of corrosion products, alloys, contamination, structure and homogenous studies. There are many companies that use reverse engineering to produce or to repair products. Determination of the base material becomes necessary. The EDS system has limitations, as it is semi-quantitative and not fully quantitative. Other tests are then utilized to fill in the unknowns. Some of these include hardness and/or conductivity testing. The material from a part in which the alloy is not known can be tested non-destructively, as only a small chip or scraping is needed. A small piece is placed into a scanning electron microscope, which magnifies up to 20,000X. Basic materials can then be analyzed to determine the alloy. In other instances, additional analysis such as microstructural and emission spectrograph can be used. These, however, are not non-destructive in nature. The analysis can reveal contamination along with identifying corrosion products. The results can be used to determine the history of the component parts. When someone has an expensive aircraft part, which they plan to reverse engineer or repair, knowledge on the composition of the alloy used is essential. The part can be scraped and a small particle analyzed. The same procedure can be followed for plating or sealants. The parts do not need to be destroyed. Some materials can present challenges for the individuals performing the analysis. Separating aluminum alloys is one of those challenges. Separation of many stainless steels and low alloy steels can also be a challenge. We have learned over the years that many alloys can be tested by using a somewhat different technique. All analyses performed at QC Metallurgical, Inc. require a minimum 1,000 counts in the spectrum for analysis. There are times that it is necessary to vary the voltage. An example of this is an analysis of aluminum alloy such as 6061. (See Figure 2 for sample scan.) The basic analysis is run at 20 KV in a takeoff angle of approximately 350. As a sample is usually a chip, the exact takeoff angle is not known. This results in a spectrum in which the lighter elements and the heavier ones can be analyzed, however, for confirmation reasons; the sample is rerun at 30 KV. The confirmation is done in order to excite the copper lines until there is an indication of a peak or no peak. If the copper is present, the alloy is probably 6061; if it’s not, then the alloy is likely to be 6063. This technique is used for many different materials, especially those containing columbium, molybdenum, lead and other elements in which the upper peaks are not excited at 20 KV. Other tests can also be used, including hardness and metallurgical analysis. The same type of analysis is used to separate many of the stainless steels, such as 316 or 347 stainless steel.
The EDS analysis technique can also be used in paint analysis. In South Florida, many structures are coated with paints which have fluorine in them. The same paint may exist without the fluorine, but they do not last nearly as long. The same could be said for Teflon materials. Aluminum alloys which have been anodized have to be sealed in order to prevent corrosion. There are many sealants which are used, including nickel and chromium. The EDS analysis is a tool used to indicate which type of sealant is being used in the samples being examined. In general, our laboratory uses ASTM A1508; a consensus standard test method. In most cases, a non-standard method is utilized. There are times when both are used, however, this requires a larger sample. In the aircraft industry, the prime contractors require a vendor to ensure that the materials they purchase are what they have actually received. The semi-quantitative analysis is used to ensure that the vendor has received what he purchased. This is a routine test used to confirm that the correct materials are being used in manufacturing of their components. The use of ED’s analysis for corrosion studies is not only common, but also essential. The EDS analysis will indicate the basic corrosion properties plus EDS can detect carbon and oxygen. These are not typically quantified, however, their presence can be important for the investigator. The EDS analysis will detect all elements, except for hydrogen, helium, lithium, beryllium and boron. Corrosion processes such as dezincification or other parting corrosion mechanisms containing aluminum or nickel can be used. Photographic methods using either dot mapping or line profile analysis can be used. These are very useful tools in corrosion analysis. Diffusion can also be shown graphically using these techniques. These methods are very useful in revealing materials that are not homogeneous. In general, the use of EDS analysis in a materials laboratory is a very important tool. This laboratory uses this type of analysis almost every day for either material identification or corrosion analysis.
|
 The energy dispersive spectroscope (EDS) is a key piece of equipment in material testing laboratories. It is an x-ray fluorescence system using energy dispersion rather than frequency (Figure 1). The unit is attached to a scanning electron microscope allowing for very small sample sizes to the analyzed. The data is semi-quantitative, but it is essential in some types of analyses.
The energy dispersive spectroscope (EDS) is a key piece of equipment in material testing laboratories. It is an x-ray fluorescence system using energy dispersion rather than frequency (Figure 1). The unit is attached to a scanning electron microscope allowing for very small sample sizes to the analyzed. The data is semi-quantitative, but it is essential in some types of analyses.