
|
Introduction The environment-friendly dye sensitized solid state solar cells (DSSC) have attracted much attention since 1991[1] due to their high efficiency and low cost production for practical applications. The past decade has seen great progress in both device fabrication techniques and material development. The typical DSSC is normally in a sandwiched configuration, filled with a HTM in the space between the dye sensitized nanocrystalline TiO2 photoelectrode and counter electrode. Under the irradiation by visible light, the dye molecules become photoexcited and on an ultrafast time scale, direct electrons into the conduction band of titanium dioxide (TiO2). Then the oxidized dye sensitizer is effectively scavenged by HTM, which itself is regenerated at the counter electrode by the passing of electrons through the external load. Usually, synthetic complexes such as ruthenium (II) complexes with carboxylated polypyridyl ligands are employed as molecular sensitizers in DSSC [2]. In order to replace the rare and expensive ruthenium compounds, many kinds of organic synthetic dyes have been actively studied and tested as low cost materials [3] for DSSCs. Some researchers have obtained good solar electric power conversion by testing natural dyes as inexpensive environmentally-friendly alternatives to artificial sensitizers for DSSCs [4]. In nature, some fruits, flowers, leaves, etc. exhibit various colors containing pigments that can be easily extracted and then employed in DSSC for either educational purposes or indoor applications. Therefore, unlike artificial dyes, the natural ones are is easily available, easy to prepare, low cost, non-toxic, environmentally-friendly and fully biodegradable. We report the fabrication and performance of DSSC using four starburst molecules which we have synthesized as HTM and red sandal dye as sensitizer. In our previous paper, the synthesis, characterization and fabrication of DSSC using N,N,N- Tris- (2-ethoxy-naphthalenen-1-yl) -N,N,N’-triphenylbenzene-1,3,5-triamine [4b] were reported. The conversion efficiency of DSSC is 0.39 [5]. The alkoxyl groups in HTM play an important role for capture of electrons so we change the alkoxyl group to improve the efficiency of solar cell. Here the HTMs are the derivatives of N,N,N-Tris-(4-butoxy-phenyl)-N,N,N-tri-naphthalen-1-yl-benzene-1,3,5-triamine are
The synthesis and characterization of N,N,N- Tris-(2-methoxy-naphthalenen-1-yl)-N,N,N’-triphenylbenzene-1,3,5-triamine[4a] were also reported [6]. Experimental MATERIALS Tetrahydrofuran (THF) was refluxed with sodium and benzophenone, and then distilled. 1, 2-dichlorobenzene was dried over CaH2 and then distilled. All reagents and solvents were purchased from Merck, Loba Chemie and SRL India and used as received unless otherwise stated. MEASUREMENTS Infrared (IR) spectra were recorded on a Shimadzu FT-IR 8400 S spectrometer as potassium bromide (KBr) disc. Ultraviolet- visible (UV-Vis) spectra were recorded from diluted solutions in spectroscopic grade ethanol on a Shimadzu 1700 UV-Visible spectrophotometer using a 1.0 cm length quartz cuvette. Proton (1H) and carbon-13 (13C) nuclear magnetic resonance (NMR) spectra were recorded on a NMR-JEOL GSX-400 spectrometer with tetramethylsilane as internal standard Using tetramethylsilane (TMS) as the internal standard and using CDCl3 as solvent in all samples analyzed. CV measurements were carried out on a Autolab potentiostat PGSTAT 12 at a glassy carbon electrode using millimolar solutions in acetonitrile (ACN) containing 0.1M of a supporting electrolyte, tetrabutylammonium hexafluorophosphate (TBAPF6), in a three electrode cell and potentiostat assembly at room temperature. The CV measurements were carried out at a glassy carbon electrode using millimolar solutions in acetonitrile (ACN) containing 0.1M of the supporting electrolyte tetrabutylammonium hexafluorophosphate (TBAPF6), in a three electrode cell and potentiostat assembly at room temperature. The potentials were measured against platinum as reference electrode and each measurement was calibrated with an internal standard, ferrocene /ferrocenium (Fc) redox system. DSC studies were performed with a NETZSCH DSC 204 thermal analyzer under inert atmosphere. The compounds are analyzed for heating and cooling thermograms (cyclic) in an inert atmosphere from -50oC to 250oC at a rate of 10oC/ min. SYNTHESIS OF 2-ALKOXY NAPHTHALENE Alkyl bromide (0.03mol) in toluene (25mL), 2-naphthol (4.32g, 0.03mol) in 40% NaOH (20mL) and tetrabutyl ammonium bromide (0.9g, 0.003mol) were refluxed at 70oC for 4.0 hours. After the completion of the reaction which is monitored by TLC, the product is extracted using toluene – water system. Scheme 1 represents the structures and scheme for the synthesis of alkoxy naphthalenes. n-propyl bromides, n-butyl bromides and n-pentyl bromides were used as alkyl bromides to obtain the corresponding alkoxy naphthalene.
2-butoxy naphthalene [1d]: 2-pentoxy naphthalene [1e]:
SYNTHESIS OF 2-ALKOXY NAPHTHALENE-1-YL-AMINE 2-alkoxy naphthalene (0.01mol) and conc. H2SO4 (5mL) were added in around bottom flask equipped with a condenser. The mixture is cooled and the temperature is maintained at 5 degrees Celsius or less. To this, conc. HNO3 (4mL) was added drop wise with stirring. The solution was stirred for one hour at 0oC, one hour at room temperature and one hour at 60oC. The contents of the flask were cooled, poured into ice and neutralized with 40% NaOH. The yellow compound obtained is filtered, washed, dried, and recrystallized from ethanol. The product (1.64g, 0.008mol), ammonium ferrous sulphate (1.5g, 0.003mol), ethanol (20mL), water (5mL) and conc. HCl (0.5mL) were heated in around bottom flask on a stem bath for one hour. The product was extracted from the mixture by washing with hot ethanol and then dried. The yellow solid thus obtained was recrystallized from ethanol. Scheme2 represents the structures and scheme for the synthesis of Alkoxy naphthyl amine.
2-butoxy naphthalene-1-yl-amine [2d]:
2-pentoxy naphthalene-1-yl-amine [2e]:
SYNTHESIS OF N,N,N’-TRIS-(1-ALKOXYNAPHTHALEN-2-YL)-BENZENE-1,3,5-TRIAMINE 2-alkoxyy naphthalene-1-yl-amine (0.03mol), tribromobenzene (3.30g, 0.01mol), CuCl (0.02g, 0.0002mol) andK2CO3 (1.0g, 0.007mol) (dried at 110oC) were refluxed together with 20 ml acetone for 10hours at 60oC. After removal of the solvent by vacuum, ammonia solution (50mL) was added and the mixture was left to stand for 2 hours. Ethyl acetate (150mL) and water (100mL) were added. The organic phase was separated, washed with water (100mL×2) and brine solution (100mL), dried over anhydrous sodium hydrogen sulfate, and concentrated to yield a yellow residue. The yellow residue was purified via column chromatography using ethyl acetate - hexane (4:1) as the eluent. Recrystallisation from ethanol yielded a yellow solid. Scheme 3 represents the structures and scheme for the synthesis of N,N,N’-Tris-(2-alkoxy-naphthalen-1-yl)-benzene-1,3,5-triamine. N,N,N’-Tris-(1butoxy naphthalen-2-yl)-benzene-1,3,5-triamine [3d]: N,N,N’-Tris-(1-pentoxyynaphthalen-2-yl)-benzene-1,3,5-triamine[3e]: SYNTHESIS OF N,N,N’-TRIS-(2-ALKOXY-NAPHTHALENEN-1-YL)-N,N,N’-TRIPHENYLBENZENE-1,3,5-TRIAMINE N,N,N’-Tris-(2-alkoxy-naphthalen-1-yl)-benzene-1,3,5-triamine(0.0071mol), bromobenzene (2.2 mL, 0.0213 mol), CuCl (0.02 g, 0.0002mol) K2CO3 (1.0 g, 0.007 mol) (dried at 110oC) were refluxed together with dichlorobenzene(20 mL) 10 hours at 170oC. The progress of the reaction was monitored by TLC. After removal of the solvent in vacuum, ammonia solution (50 mL) was added and the mixture was left to stand for 2.0 hours. Ethyl acetate (150 mL) and water (100 mL) were added. The organic phase was separated, washed with water (100 mL×2) and brine solution (100 mL), dried over anhydrous sodium hydrogen sulfate, filtered and the solvent is removed in vacuum. The product was purified via column chromatography using ethyl acetate – hexane as eluent to obtain a yellow solid which is recrystalized from hexane. Scheme 4 represents the structures and scheme for the synthesis of N,N,N-Tris-(2-alkoxy-naphthalenen-1-yl)-N,N,N’-triphenylbenzene-1,3,5-triamine. N,N,N- Tris- (2-butoxy-naphthalenen-1-yl) -N,N,N’-triphenylbenzene-1,3,5-triamine [4d]:
N,N,N- Tris-(2-pentoxy-naphthalenen-1-yl) -N,N,N’-triphenylbenzene-1,3,5-triamine[4e]: DEVICES FABRICATION DSSC device comprised of a transparent conducting oxide (TCO) glass electrode coated with porous nanocrystalline TiO2 (nc-TiO2), dye molecules attached to the surface of the nc-TiO2, hole transporting material and a counter-electrode. Fluorinated tin oxide (FTO) coated transparent glass strips (25mm x 25mm) with resistance of 30μ ohms were used for the device fabrication. The structuring of the TCO is done using a chemical etching method. Zinc granulates are spread out on the glass (20 mg/cm2) and reacted with 4 N HCl (1 mL/cm2). Scotch tape is used to mask the TCO area needed for the back contact. The fast reaction between HCl and Zn leads to the removal of the SnO2. After two treatments of 3 minute reaction time, theSnO2 is completely removed. The structured glass is then cleaned by ultra-sonification in various solvents, such as acetone, ethanol and water, for 10 min in each solvent. Compact TiO2 (30 nm) were deposited on fluorine-doped SnO2 (F-SnO2) glass by spin coating that acts as a blocking layer. The FTO is placed in a chamber for 1 min and accelerated at a speed of1000 rpm. 150 μl of TiO2 solution is applied onto the substrate. The substrate is spun up to a speed of 1000 rpm for 30 s with an acceleration of 200 rpm/s. The samples are dried for 30min. After deposition, the prepared TCO/TiO2 was annealed at 100°C for 1 hour in air with a hot plate to achieve complete pyrolysis of organic species. Then nanoporous TiO2 (~2.5μm) film was deposited by tape casting method. The layers were sintered for 30 min at 350oC to consume the organic additives and to obtain mechanically stable samples. Then the sample is cooled down slowly to 80oC and placed into alcoholic solution of red sandal dye overnight. After, the dip coating, HTM in THF (~800nm) deposited on the cell by spin coating method. The organic cells were kept overnight to allow maximum penetration of HTM in TiO2 . The metal electrode silver (200nm) is coated on HTM of DSSC by thermal evaporation. Result & Discussion The synthetic strategy employed for the synthesis of N,N,N-Tris-(2-alkoxynaphthalenen-1-yl)-N,N,N’-triphenylbenzene-1,3,5-triamine derivatives are described in scheme1. Since the synthesis of the final compound involves multistep organic reaction adding two more wings to the core benzene ring, the overall yield of the synthesis is very low. THERMAL PROPERTIES For solar cell application, the thermal stability of the organic material is crucial for device stability and lifetime. The glass forming properties and phase transition of compounds (4a, 4c, 4d and 4e) are studied using DSC. The compounds are analyzed for heating and cooling thermograms (cyclic) in an inert atmosphere from -50oC to 250oC at a rate of 10oC/min. These five compounds differ only in their substitutions at outer naphthalene rings. Due to these differences, we expect only a very few changes in thermal properties. Accordingly, the solubility and density of the compounds may also vary. The Tg, Tc and Tm values of all synthesized compounds are listed in Table 1. From these data, we recognize that there is only a very small change in thermal properties. This may be due to the change of substitution at the outer naphthalene ring. Well-defined glass transitions are at 65, 59, 50 and 46oC, respectively. Tm values of compounds (4a, 4c, 4d and 4e) are at 95, 70, 60 and 59oC, respectively. All the above compounds are decomposed above 250oC. The compound 4a shows two-phase transitions at 95oC and 115oC. The two transitions in DCS give the possibility of a liquid crystalline nature for the compound.
ELECTROCHEMICAL PROPERTIES The electrochemical stability is one of the other main conditions to be fulfilled by the materials to find application in various electro-optical devices. The results of electrochemical studies are listed in Table 2. The electron affinity (EA), ionization potential (IP) and band gap of the compounds are calculated from the data. The high-lying HOMO energy level and reversible electrochemical oxidation of 4a, 4c, 4d and 4e suggest that these compounds have high potential for hole transporting material.
DEVICE PROPERTIES The result for photocurrent density (ISC), open-current voltage (VOC), fill factor (FF) and corresponding photoenergy conversion efficiency (η) are summarized in Table 3. The efficiency of compound 4b is 0.39 [5]. Different HTM exhibits variable photoconversion efficiencies that are 0.2, 0.7, 0.7 and 0.72 for 4a, 4c, 4d and 4e respectively. Compared with this efficiency, efficiency of 4a is lower and 4c, 4d, 4e are higher. From the result, we can say that as the length of alkyl group increases, the efficiency increases. The low efficiency could be attributed to the poor pore filling of the hybrid nanoporous TiO2/dye film by the organic HTM, which induces poor dye regeneration and hence low photocurrent.
Conclusion All five compounds are capable of functioning as DSSC. The efficiency of the devices varies from 0.2% to 0.72%. This may be due to the difference in length of the alkyl group. From these results, we can conclude that as the length of alkyl group increases the efficiency increases. Instead of the ruthenium dye and back electrode gold, we have used the red pigment of red sandal and silver. These are the two major differences we adopted. The low current values we obtained may be due to layer thicknesses of different materials and the possible degradation of natural dye. In the meantime we would like to emphasize the total cost effect of the cell, which will be down to nearly 30-40% of a similar type fabricated cell. The conversion of the non-conventional energy, even if for a very low percentage, is advancement to mankind. The use of a reduced amount of chemicals is an added advantage when the natural dye is applied. This will be an introductory step to the green synthesis. Further studies are being carried out for the optimization of layer thickness of each material and use of different natural dyes to improve the efficiency of DSSC. References
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
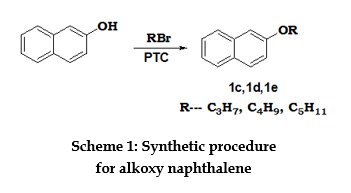 2-propoxy naphthalene [1c]:
2-propoxy naphthalene [1c]: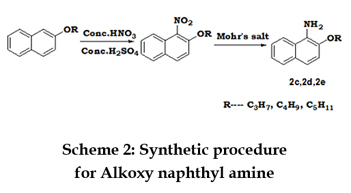 2-propoxy naphthalene-1-yl-amine [2c]:
2-propoxy naphthalene-1-yl-amine [2c]:
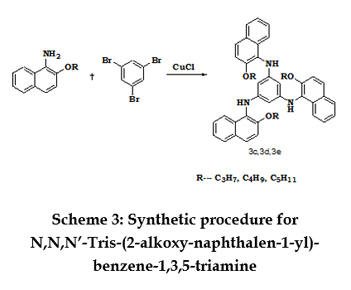 N, N, N’-Tris-(2-propoxy-naphthalen-1-yl)-benzene-1, 3, 5-triamine [3c]:
N, N, N’-Tris-(2-propoxy-naphthalen-1-yl)-benzene-1, 3, 5-triamine [3c]: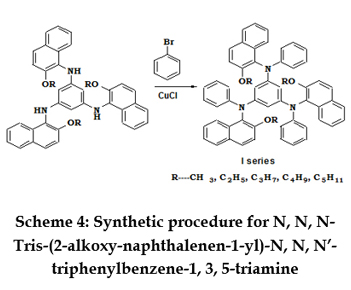 N,N,N-Tris-(2-alkoxy-naphthalenen-1-yl)-N,N,N’-triphenylbenzene-1,3,5-triamine[4c]:
N,N,N-Tris-(2-alkoxy-naphthalenen-1-yl)-N,N,N’-triphenylbenzene-1,3,5-triamine[4c]: