|
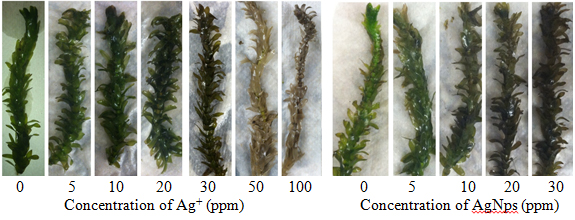
Introduction Silver Nanoparticles Nanotechnology is the study of chemical and physical interactions on the scale of 1-100 nm, a size range in which matter possesses distinct properties from both individual atoms and bulk particles. Nanomaterials are used for medicinal applications, environmental remediation, consumer products, and electronics. Silver nanoparticles (AgNps) in particular have been incorporated into 24% of almost 2000 consumer products containing nanoparticles [1]. They have diverse applications and are becoming more common in industrial processes. Small AgNps (<10 nm) can be more toxic to bacteria relative to Ag+ because of differences in size, shape, surface properties, and coating characteristics [2]. This enhanced toxicity has led to the increased use of AgNps as an antimicrobial agent. The increased fabrication and use of AgNps poses threats to aquatic ecosystems. Although the U.S. Environmental Protection Agency (EPA) has regulated Ag+ release since 1980, the growing use of AgNps over the past 10 years requires that the environmental impacts of these particles be thoroughly examined to determine their long-term effects on our waterways and aquatic ecosystems. Sources of silver pollutants include waste from natural leaching, mining, and the photography industry. While silver salt discharges into the environment have decreased, silver toxicity remains a concern due to their increased use in consumable products. The fate of AgNps has been estimated based on models in aquatic systems, and indicate adverse effects depending on natural organic matter concentrations, ionic strength, and electrostatic effects between nanoparticle coatings and water composition [3]. For example, the oxidation of AgNps can also contribute to silver cation concentrations in water. The presence of silver species in the environment, particularly the water supply, can result in bioaccumulation across multiple trophic levels with potentially toxic effects. While plants can be used for phytoremediation of silver species, the effects on fish and other food sources can be devastating to the food chain. The EPA regulates total silver in aquatic environments, ensuring concentrations are 1.2-13 ppb (depending on CaCO3 concentration) to limit the impacts on an ecosystem [4]. In addition, aquatic ecosystems are prone to silver contamination because of its increased uses manufacturing items such as soaps, textiles, and plastics [5]. While accidental discharges of silver compounds have declined after the 1970s, it is still estimated that 2500 tons of silver are released into waterways each year and that 80 tons ends up in surface waters [5, 6]. Silver nitrate is the most toxic form of silver because of its dissociation into Ag+. Other silver species have been identified as less toxic because they are largely insoluble, including complexes with chlorides and sulfides [6]. Increased understanding of nanoparticle behavior has led to greater focus on the effects of these particles on ecosystems. Mechanisms of Silver Species Toxicity Silver nanoparticles and Ag+ have distinct mechanisms of toxicity but both are lethal to a variety of organisms, including bacteria, plants, animals, and fish in aquatic ecosystems [5, 7]. Silver species can damage the cellular structure and organelles through unfavorable binding interactions [8]. Further, size-based translocation of silver species is attributed to differences in toxicity between Ag+, insoluble silver salts, and AgNps [5, 6]. Silver cations enter the cell through transmembrane sodium and copper ion transporters and can accumulate in organisms [5]. These cations inhibit respiratory enzymes, induce oxidative stress, and bind to molecules containing sulfur and phosphorous [8]. Silver species exhibit a strong affinity for thiol groups on proteins and enzymes, causing the inactivation of these macromolecules due to formation of Ag-S bonds [9]. Two methods of translocation into the cell have been proposed. The first is by the association of the nanoparticle to the cell wall and release of Ag+ [5]. Silver cations released by the AgNps cause damage through interference with the mitochondria, as seen in toxicity experiments with Lemna gibba (duckweed) [10]. Since AgNps can attach to the cell wall, any Ag+ released due to the oxidation of the nanoparticles has a greater likelihood of entering the cell. In the second mechanism for toxicity, AgNps enter the cell through endocytosis, caused by the invagination of the plasma membrane. Vesicles form and disperse particles throughout the cell. Transmission electron microscopy (TEM) images of plant seedlings have demonstrated particle translocation in plants [11]. The association of AgNps with the active sites of proteins has been observed in the bacteria Escherichia coli and suggests that, depending on the coating, nanoparticle-protein association can decrease or inhibit enzyme and protein activity [12]. Phytoremediation of Heavy Metals Phytoremediation is a green method used to remove contamination from terrestrial and aquatic environments based on the absorption and accumulation of heavy metals or toxins by plants [13]. Aquatic macrophytes are used for phytoremediation of toxic metals and E. densa effectively removes heavy metal cations from water. There are diverse applications of phytoremediation in aquatic plant systems, including re-harvesting toxins in plants unaffected by heavy metal uptake (phytoextraction) and absorption/adsorption of heavy metals by plant root systems (rhizofiltration). Both applications remove contaminants from the ecosystem. Humic Acid Reduction of Ag+ to Form AgNps The reduction of AgNO3 to AgNps in the presence of humic acids has been reported and is well characterized [14-16]. Humic acids are large polymers composed of organic structures found in soils and other geological features. Structures of humic acids vary by sampling location, but common characteristics include aromatics, heterocycles, carboxyls, and nitrogen content [17]. Humic acids used in these experiments are from the Suwannee River (GA), and the samples are generally consistent in the composition of the constituent functional groups and components [18]. Suwanee River humic acids have the following elemental composition: 52-53% C, 4-4.5% H, 42-43.5% O, 0.5-0.75% N, 0.4-0.6% S, and trace amounts of P. Humic acid reduction of Ag+ yields nanoparticles that remain stable for approximately 2 months, based on spectroscopic measurements [14]. This reduction indicates that AgNps may form under environmentally relevant conditions. Many acute toxicity studies of AgNps have been performed using methods that assume ideal accumulation, dispersion, and suspension [16]. While useful, these conditions are not environmentally relevant. E. densa is an aquatic macrophyte used in phytotoxicity tests and pollution modeling due to its ability to accumulate contaminants and heavy metals. No studies have been conducted on the ability of the waterweed, E. densa,to absorb and remove silver species from an aqueous environment. This study investigated the absorption of Ag+ and AgNps by E. densa as part of a larger study of silver toxicity towards plants. Experimental Chemicals All chemicals were reagent grade and used as received. Plants were grown in synthetic moderately hard reconstituted freshwater (MHRFW) as previously described [19] during treatments. Suwannee River Humic Acid Standard II was purchased from the International Humic Substances Society (IHSS, St. Paul, MN). Egeria densa was purchased from Nahackey’s Aquarium (Melbourne, FL) in January 2015 and grown in reverse osmosis water for at least 2 days before testing. Silver Nanoparticle Synthesis and Characterization Plant Material and Metal Treatment Silver nitrate was used to prepare 5, 10, 20, 30, 50, and 100 ppm Ag+ in MHRFW. A stock silver nanoparticle solution in MHSFW (30 ppm AgNps) was used to prepare silver nanoparticle treatment solutions at 5, 10, 20, and 30 ppm AgNps. Control solutions consisting of MHSFW and 15 ppm humic acid in MHSFW were prepared for the Ag+ and AgNps studies, respectively. Stalks of E. densa (12 cm in length) were grown in test tubes filled with 50 ml of treatment solutions. Plants were grown in solution for 1 week in the Florida Institute of Technology greenhouse in February 2015. Temperature was maintained at approximately 250C. Solution levels were topped off with DI water every 2 days to ensure a constant volume of treatment solution. Silver Analysis Statistics Results & Discussion Analysis of Synthesized AgNps An absorbance spectrum of the humic acid-reduced AgNps showed a peak at 415 nm and an absorbance of 1.17. This compared favorably to the spectrum reported by Akaighe [15]. The concentration of nanoparticles was determined to be 30 ppm with an average particle diameter of 30 nm. Thus, these particles can enter the cell through pores in the plasma membrane or by endocytosis, which involves the invagination of the plasma membrane to absorb small particles and subsequent translocation throughout the cell [5]. Visual Analysis of Silver Species Toxicity A decline in health was evident in plants exposed to silver in concentrations as low as 5 ppm. Plant health was generally hindered at concentrations of 20 ppm Ag+ and AgNps, as seen by the significant browning of the leaves in Figure 1. Higher concentrations of silver had a more pronounced effect on the plants for all measurements. Therefore, the presence of Ag+ or AgNps caused an obvious decline in plant health over the exposure period.
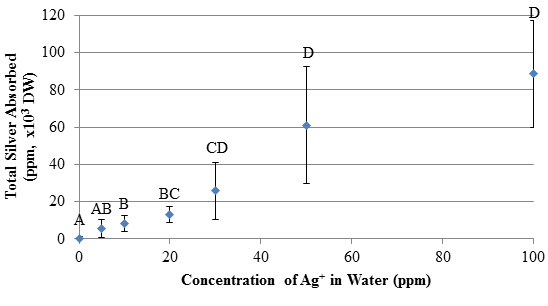
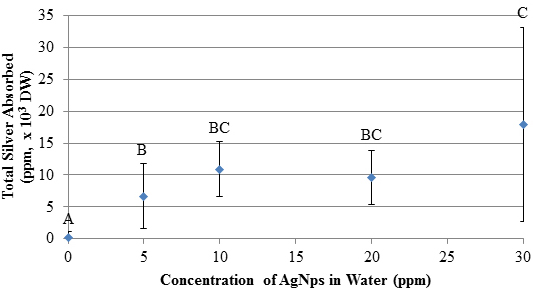
Similar phenotypes have been observed in related waterweeds such as Elodea canadensis, and have been linked to insufficient chlorophyll production by leaves (chlorosis) and cell death from toxic environments and extracellular factors (necrosis) [23, 24]. In these studies, 100 ppm lead exposures caused the leaves to turn brown and E. canadensis underwent chlorosis [23]. Similar effects were observed in the E. densa tests presented here with Ag+ at 50 and 100 ppm. The plants underwent significant changes in color, resulting from silver toxicity which caused chlorosis and necrosis. Further, E. densa leaf tips began to turn brown at low concentrations of silver, indicating phytotoxicity to the photosynthetic apparatus and other subcellular components. As silver was absorbed, the E. densa withered and died. This is caused by an oxidative burst in the cell, resulting in lipid peroxidation, degradation of macromolecules, and other unfavorable reactions [5]. Silver Absorption Analysis Results of the AgNps absorption study are shown in Figure 3. Absorbed silver increases with exposure to increasing concentrations of AgNps. (It should be pointed out that flame AA detects silver but does not distinguish between nanoparticles and cations released by the nanoparticles.) Like the Ag+ results, the AgNps demonstrate a positive, dose-dependent increase in total silver absorption (r=0.711, p<0.001). Measurements of absorbed silver in the plant when exposed to 30 ppm AgNps solutions are statistically different compared to absorbed silver due to a 5 ppm solution. Solution concentrations of 10 and 20 ppm yield intermediate results. Comparisons of results for similar solution concentrations for the two systems show no differences in absorbed silver (p>0.05). This indicates that both Ag+ and AgNps are absorbed to an equal extent by E. densa. Further, this similarity supports conclusions that the humic acid reduction of AgNO3 produces AgNps which are as toxic as Ag+ at the same concentration. Concerns about ethical disposal of AgNps and Ag+ -containing products are validated by these results.
Bioconcentration Factor (BCF) In this study, total silver species bioconcentration was measured, as shown in Table 1. High BCF values indicate that E. densa absorbed significant amounts of silver from its environment. The bioconcentration factors for the AgNps show a general decrease as concentration of AgNps in solution increases, whereas the Ag+ BCF is relatively constant. In both cases, silver species were absorbed into the plants. These BCF values of E. densa are reasonable compared to those in the literature. There was a significant decline in BCF values for AgNps (r=-0.56, p<0.001) at higher AgNp concentrations. The amount of absorbed silver in plants at higher AgNp solution concentrations is greater than at lower concentrations but the amount absorbed does not increase proportionally to the AgNp solution concentration. The inability of silver to enter the plants at higher AgNp concentrations may be caused by some nanoparticles blocking the preferable adsorption sites on the surface of the plant. Alternatively, the nanoparticles could be involved in another step in the process of the plant’s uptake of silver which has a rate that is independent of AgNp concentration. The significant decline in BCF for AgNps supports this, as plants exposed to 10-30 ppm AgNps showed no significant differences in total silver absorption (shown in Figure 3), suggesting that relatively fewer AgNps were absorbed by the plants when exposed to higher AgNp concentrations. The BCF values for Ag+ exposure were independent of Ag+ concentration (p>0.05), meaning that free Ag+ is readily absorbed by E. densa regardless of the concentration in solution,even after the plant shows severe signs of degradation at 50 and 100 ppm Ag+.
E. densa absorbs similar amounts of Ag+ and AgNps based on the bioconcentration factors of the two systems having the approximately the same values. Visual analysis of the plants suggests that like concentrations generally have the same effect on the plants. These results do not support one mechanism of silver toxicity over another, but clearly show that Ag+ and AgNps are both toxic to E. densa. Conclusions AgNps were successfully synthesized and characterized according to the humic acid reduction of silver nitrate. Visual effects of Ag+ and AgNps were observed at the end of a 7 day exposure of E. densa. Exposures to either silver cations or AgNps resulted in the bioaccumulation of silver within the plant cells and the amount of absorbed silver was proportional to the concentration in solution. Bioconcentration factors showed that plants absorbed significant amounts of silver species from the aqueous environment, indicating that E. densa is a viable candidate for phytoremediation of both types of silver. These results are similar to studies involving other waterweeds, and demonstrate the negative effects of heavy metal pollutants on plants. Silver bioconcentration factors were of the same order of magnitude for both Ag+ and AgNps, but BCF declined at higher concentrations of AgNps while BCF of Ag+ remained constant. This suggests that the nanoparticles have a dependency on free Ag+ in the water. Further, the higher accumulation of Ag+ caused significant declines in physiological features and their subsequent activities, indicating that silver species are toxic to E. densa. Measurements of the total absorbed silver and BCF values indicate that E. densa can be used for effective phytoremediation of areas contaminated with silver species. Heavy metal contamination is a growing environmental concern, so successful removal using phytoremediation approaches with duckweeds and waterweeds should be studied further to provide an environmentally benign solution to remove toxins entering waterways. Additional studies can be performed using macrophytes and similar organisms (i.e. duckweeds) to determine which is most effective for silver species phytoremediation. References
|
|||||||||||||||||||||||||||||||||