|
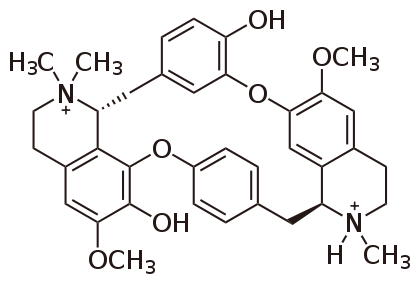
Background From prehistoric times to the present, people have used (and abused) toxic chemicals. A few [1] of the many toxicological landmarks might include: a) The Ebers Papyrus (c.1500 B.C.) which described many known poisons of that time, b) The Book of Job (c. 400 B.C.) which described poison arrows, c) Hippocrates (c. 400 B.C.) who described some therapeutic uses for toxins, d) Theophrastus (c. 300 B.C.) who made a record of poisonous plants, e) Sulla (c. 82 B.C.) who wrote the first law against poisoning, f) Dioscorides (c.60 A.D.) who made a classification of plant, animal, and mineral toxins, g) Maimonides (c. 1200 A.D.) who wrote a paper on the treatment of poisoned patients, h) Catherine de’ Medici (c. 1580 A.D.) who kept a diary in which she recorded dosages, symptoms, time frames, and complaints of people she experimented on with different poisons, i) Paracelsus (c. 1567 A.D.) who published a treatise on the occupational cause of miners’ disease, j) Percival Pott (c. 1775 A.D.) who published a paper on the carcinogenicity of soot (polyaromatic hydrocarbons) in chimney sweeps, k) Orfila (c. 1825 A.D.) who developed forensic chemical tests for poisons and used them in legal cases, l) Schmiedeberg (c. 1880 A.D.) who trained numerous toxicology students who then worked in pharmacology and toxicology laboratories, m) the introduction of laws governing the use and regulation of anesthetics, disinfectants, patent medicines, drugs, food additives, cosmetics, pesticides and fungicides (c. 1850-present), n) the developmental birth defects among the offspring of mothers who had used thalidomide (c. 1960s) which led to legislation banning the use of the drug and requiring pharmaceutical industries to do multi-species animal testing on drugs prior to marketing them, and o) the publication of Silent Spring by Rachel Carson (c.1962) which led to laws governing the use of environmental toxins and toxicants. It should also be noted that wars have led to the development and use of toxic chemicals for military gain. A few 20th century examples [2] would include chlorine, mustard, tabun, sarin, soman, and lewisite gases, radioactive chemicals such as uranium and plutonium, and biological agents such as Y. pestis and V. cholerae. New agents (e.g., Agent Orange) and old ones (e.g., rye ergot), they have all made their mark on history. During the 16th to the 18th century, history was riddled with the use of poison by a number of prominent individuals for a host of reasons spanning from infidelity to inheritance. Two of the most ruthless poisoners included the Marquise of Brinvilliers, whose exploits were so astounding that famous author Alexandre Dumas wrote a novel based on true events surrounding her life [3-4]. This "Lady of Poison" was proven to have poisoned the majority of her family for the family inheritance [4]. However, she first tested her poison on the patients of the surrounding hospitals to test the potency of the poison before administering the poison to her family [4]. She is even rumored to have poisoned her very own daughter for the crime of being a simpleton, though she did instantly regret the act and quickly administered the antidote … milk [4]. Upon the death of her lover, a military man and ex-convict, Sainte-Croix, a chest was found containing correspondence between himself and the Marquise that told of all her exploits [4]. The Marquise was then captured, tortured, tried, beheaded and burned in 1676 [4]. It was said that the crowd that had gathered to witness her burning danced in her ashes as they blew over the crowd [4]. Another woman of renowned talent in the art of poisoning was Catherine Deshayes [4-5]. She was nicknamed "La Voisine," and at the time of her death she had confessed to a multitude of poisonings of jealous or unfaithful husbands, wives and lovers. She even confessed to the murders of many unwanted children birthed by unwed women [5]. She boasted of having poisoned more people than all the famous or professional poisoners of her era [5]. However, poisoning was not Deshayes' only method of killing unwanted children [5]. She was known for her practice in the dark arts, the occult, and Satanism [5]. It was rumored that if an unwed woman were to conceive, Deshayes' services would be given to the woman free of charge and would encompass free room and board while the woman carried the child to full term [5]. Upon the child's birth, Deshayes would then sacrifice the child to her deity [5]. It was even whispered that her stepdaughter was her apprentice and took part in the ceremonies [5]. Interestingly enough, the same official responsible for the capture, torture and justice of the Marquise de Brinvilliers in 1676, Officer Desgrez, was also the driving force behind Deshayes' downfall as well [5]. Deshayes was arrested and held in prison until all the aristocracy that had any connection with her were under protection, at which time she was then tortured and burned in 1680 [5]. She did not go gracefully into that good night. She died cursing all the aristocracy and their hypocrisy as well as all the accusing priests [5]. The big players of this poisoning era and probably the most renowned, were all members of the same family, de’ Medici. Though the member most remembered for the family's talents with poisoning is Catherine de’ Medici, the entire family partook of the dark art and often made their living from their knowledge of the art [3,6]. Her father, the Grand Duke of Cosimo I de’ Medici aided in the assassination of Piero Strozzi by sending an aid the poison, along with instructions, to poison Strozzi's wine bottle in 1548 [6]. Ferdinando de’ Medici, Cosimo's son, is rumored to be connected to his own brother's, Francesco I de’Medici, and his wife, Bianca Cappello's, death in 1587 in order to gain the Granducal throne [6]. Though originally, the two were declared to have died from malaria, Francesco first followed by his wife a few days later, modern day technology has allowed for bone testing for arsenic and proved that the "grand-ducal couple" did in fact die from arsenic poisoning and not malaria [6-7]. Though, what can be expected when after the death of the couple, Ferdinando was placed in charge of the autopsy and all examinations and investigations into the couples death [7]? Then there was dearest Catherine, who, in truth, depending on whose account you read, tells two completely different sides of the same coin. This rampant use of poisoning for political reasons, personal vendettas and power struggles had everyone of that era on edge. Kings were being warned that their own children were planning on not only poisoning them, but also their wives and all remaining children [6]. Queen Elizabeth I herself became the focus of a failed poisoning attempt from one of her own ladies-in-waiting [6]. The royal cook threatened to poison the food of King Henry IV of France and Maria de’ Medici herself [6]. The threat of poisoning was everywhere. There is even one story of a minister of Spain becoming so paranoid that one night his steward did not rinse out his wine glass well enough to rid the glass of residual vinegar and salt giving the wine an odd taste sending the minister into a crazed panic and yelling for antidotes [6]. Even Cosimo I de’ Medici himself was threatened to be wary of his own face towels [6]. While another of the de’ Medici family, Giangiacomo de’ Medici di Marignano, was also the target of a successful assassination via poisoning [6]. Though the de’ Medici’s style of poison and antidotes mainly stemmed from herbology, it was not the only form of that time. There was a case in 1568, where a fourteen year old girl ground up mercury mirror glass into a fine powder and seasoned her family's salad with the powder all because her parents were going to send her to a convent [6]. Also, not all poisons and antidotes came from the de’ Medici family. In 1660, there was a Roman priest that used the poisons and antidote from a woman known as Poisonous Girolama in order to make his "miraculous cures" seem more fantastical [6]. Though all poisons known and utilized during this era are not all plant derived (i.e., arsenic, which is a metal based poison), herbology was the main practice for deriving poisons and many could be found right outside, even homegrown. Included in this paper only a scant few of the deadly poisons derived from plants will be discussed. Though some may not have been used in the de’ Medici era of poisoning, it is quite possible that some actually were. For example, "a description of ‘horribly deformed corpses’ and ‘profuse bleeding from all orifices’" leads one to wonder, could strychnine poisoning have been the culprit [6]? Poisons Found in the Wild Curare Plants The plants containing the curar toxin reside in the Loganiaceae and Menispermaceae families [8]. These ‘curare plants’ contain a toxin known as tubocurarine (Bowman, 2006). This toxin resides in all parts of the plant and is obtained by boiling the roots, bark and stalks of the plant [8]. Two species of plants are used to isolate tubocurarine: Chondrodendron tomentosum and Strychnos toxifera [9]. This toxin is a member of the quaternary alkaloids (meaning it is an alkaloid structure that contains four R groups branching from its core structure) and acts as a neuromuscular junction blocker [8]. Though one of the R groups of the alkaloid contains one nitrogen that is tertiary, this nitrogen is charged at physiological pH and therefore keeps the classification of the molecule as a quaternary alkaloid [9]. This toxin was first studied by Claude Bernard in the mid-19th century, but the South American tribes and the Amazonian tribes had been using this toxin for centuries to poison the tips of their arrows for hunting [9]. The amount of toxin used was defined by how many trees a monkey could climb or swing to after poison exposure [9]. If the monkey could climb a tree and swing to two more trees it was considered a weak dose of toxin, but if the monkey was paralyzed while climbing up the initial tree, then it was considered to have been injected with a strong or potent dose [9]. Tubocurarine is highly potent when injected into the blood stream and has very little absorptivity if ingested, which is why the tribes can still eat the meat killed in this manner and not poison themselves in the process [9]. However, this toxin is actually used in present day as a paralytic before certain types of surgeries for intubation, a treatment for tetanus, polio, spastic cerebral palsy and myasthenia gravis [9-10]. Tubocurarine is considered to be classified as a nondepolarising blocking toxin classified as a leptocurare [9]. In essence this poison blocks the transmission or communication of the acetylcholine receptors on the postjunctional face of the motor endplates of striated muscles. One study shows tubocurarine to not only block acetylcholine from interacting with the acetylcholine receptors, but to also block muscular responses to nerve stimuli [9]. This blocking mechanism is strong enough to continue to block this acetylcholine/receptor interaction such that even when a stimulant was introduced into the system, the blocking mechanism remained strong [9]. This potent blocking mechanism is believed to be contributed to the actual structure of the compound [9]. Tubocurarine's structure is shown in Figure 1.
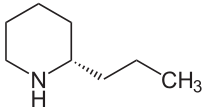
This structure is what one study believed to be a perfect example of the relationship between quaternary ammonium function and neuromuscular blocking ability [9]. The compound contains two positively charged nitrogens, even though one is a tertiary amine, giving the structure two quaternary nitrogen centers which is a concept has been thought to lead to more potent compounds [9]. This stoichiometry allows for a more flexible structure which is what this particular study believes to be a major factor in tubocurarine’s ability to bind with strong affinity and secure the receptor from binding with acetylcholine [9]. It out binds acetylcholine because that second positively charged nitrogen center will bind to another site close to the receptor (an accessory site) that plays no role in acetylcholine binding, giving it an increased chance of securing the receptor nearby [9]. This second type of receptors is termed nicotinic receptors [9]. These types of receptors are blocked by reversible neuromuscular blocking toxins (i.e., nondepolarising toxin) [9]. These receptors are found on the motor nerve endings [9]. These receptors are selective for Calcium when in their open state [9]. These nerve endings are referred to as nerve terminal autoreceptors [9]. These autoreceptors are believed to have a role in mobilizing acetylcholine from reserves in order to handle an increased frequency of nerve impulses associated with transmissions needed for striated muscle excitement [9]. Tubocurarine has been shown to block these autoreceptors, creating a ‘tetanic fade’ [9]. This tetanic fade is defined as the muscle's inability to maintain a muscle contraction no matter how high the electrical impulse generated [9]. This inability to maintain a contraction means that the muscle is at a constant relaxed state (i.e., paralysis) [9]. The effects of tubocurarine can be counteracted via acetylcholinesterase inhibitor administration or an induced dose, production, or release of increased levels of acetylcholine [9]. Under the workings of the above mechanisms, if de’ Medici or one of the other royal ladies of the court had used tubocurarine on one of her unsuspecting guests, they would have first witnessed a paralysis of the victim's eyes, nose and neck. Paralysis of the victim’s limbs would have followed. The last remaining and arguably the most important muscle to become paralyzed under the tubocurarine influence would be the diaphragm, leading to respiratory distress, which would have ceased the victim's breathing resulting in death via asphyxiation [8, 10]. Poison & Water Hemlock Though it is assumed that "a hemlock plant ... is a hemlock plant ... is a hemlock plant", in the parsley family Apiaceae/Umbelliferae, this method of logic is not necessarily true [10-13]. Yes, both poison hemlock and water hemlock are highly poisonous, but the actual toxins contained in the two different plants differ. The two plants also differ in which part of the plant contains the toxin(s). Poison hemlock’s toxins are throughout the entire plant, making the entire plant poisonous whereas water hemlock holds its toxin in the roots and stem (tubers) of the plant, making just those parts of the plant toxic with the tubers being twice as toxic as the roots [12-13]. Poison hemlock (Conium maculatum), which was used to kill Socrates in 399 B.C. in Athens, to poison political prisoners in ancient Greece, and believed to have possibly been given to Jesus Christ along with vinegar and myrrh at the time of His crucifixion, contains eight alkaloids, but there are two dominant piperidine alkaloid players: coniine (see Figure 2) and γ-coniceine [10-13]. These two toxins are formed from an eight-carbon chain from four acetate units that become cyclical [11].
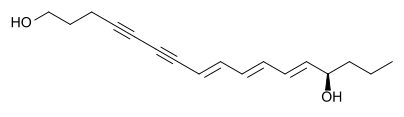
Coniine is more prominent throughout the plant during the seed and mature stages of the plant's life cycle and is the toxin most commonly found in dried poison hemlock [12-13]. Γ-coniceine is the dominant toxin present during the plant's vegetative stage of life and is tested to be eight to ten times more toxic than coniine [12-14]. It is believed that as poison hemlock dries out, there is an alkaloid "shift" from γ-coniceine to coniine [14]. These two toxins are so potent that an individual can receive a fatal dose by simply eating an animal that has ingested the two toxins [13]. The mechanisms of these two alkaloids are also nondepolarising neuromuscular blockers like their sister tubocurarine [15]. Though the effects of these two toxins are two fold, as tubocurarine is, their main focus is on the nicotinic receptors on the motor nerve endings and by blocking spinal reflexes via how they affect the medulla [11,15]. These two toxins also affect the autonomic ganglia and can overstimulate cholinergic receptors, which is why these sister toxins are considered to be biphasic in that there exists two distinct phases of reactions of the central nervous system to the alkaloids [15]. The first effect causes a stimulation of the skeletal muscles during an early nicotinic effect which is considered the initial central nervous system stimulation [12]. This stimulation is then followed by a series of seizures of progressively intense constrictions with intermediate periods of relaxation with each period of relaxation being of shorter duration than the previous [12]. This series of seizures is then followed by a relaxation of these same muscles due to the blocking of the nicotinic receptors which is the later or delayed relaxation of the central nervous system [11-12]. Poison hemlock poisoning can also lead to muscle damage which explains the raised values of muscle enzymes (lactic dehydrogenase, aspartate aminotransferase and creatine kinase), elevated liver function values and myoglobinuria [12, 15]. One study theorized that the biphasic nature of the sister toxins could actually be dose dependent. For example, at low toxic doses, a blocking of spinal reflexes by the toxins and their effects on the spinal cord cause the initial stimulation and then the secondary phase, the relaxation action, is due to the toxins' effect on the autonomic ganglia [12]. Then at larger doses, the initial stimulation phase stimulates the skeletal muscle and the relaxation phase is cause by the subsequent neuromuscular blocking mechanism [12]. Each responsive phase of poison hemlock poisoning comes with its own set of symptoms. The symptoms of the initial phase of stimulation (effects of autonomic ganglia interaction) display within fifteen to sixty minutes after ingestion and consist of: nausea, vomiting, frothing, nervousness, bronchorrhoea, hypertension, tachycardia, agitation, ataxia, confusion, muscle fasciculation, burning of the mouth, throat and abdomen, excessive salivation, trembling, loss of coordination (stumbling), dilation of pupils, mydriasis, impaired consciousness and myalgias [11-15]. The symptoms of the secondary phase of relaxation (effects of overstimulation of cholinergic receptors) consist of: diarrhea, aponea, bradycardia, hypotension, muscular weakness, muscle paralysis, lethargy, weak or slow heartbeat, headache, ataxia, coma, rhabdomyolysis, acute tubular necrosis and respiratory muscle paralysis due to phrenic nerve paralysis, often defecation and urination, and “mousy” odor to the breath, urine and sweat and eventually death from respiratory paralysis [11-15]. Poison hemlock toxins are also considered to be teratogenic toxins. This means that low constant doses of poison hemlock toxins during the gestation period of fetal development will cause deformities and abnormalities in a fetus [11-15]. These birth defects include arthrogryposis, scoliosis, torticollis, excessive flexure of the carpal joints and cleft palate [12]. This teratogenic property is believed to be due to the length of the side chains and the amount of unsaturation of the carbon bindings in the piperdine rings of coniine and γ-coniceine. Teratogenicity is usually associated with a saturated ring and a propyl or longer side chain [12]. At present there is no counteracting therapy for poison hemlock poisoning. The only aid to those unfortunate enough to ingest the toxic plant is gastric suction, administration of activated charcoal if the patient is not yet comatose, and diuresis to prevent possible renal failure due to rhabdomyolysis and myoglobinuria [11, 15]. Water hemlock (Cicuta verosa), though from the same family as poison hemlock, contains cicutoxin, which is a toxin that acts directly on the central nervous system, but is not an alkaloid [11, 15]. This toxin is a long double and triple bonded (i.e., highly unsaturated) carbon chain alcohol and is shown below in Figure 3. This toxin is believed to be the most violently poisonous of all toxins [11-12].
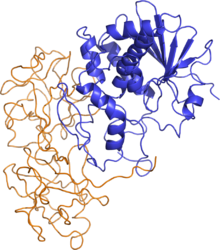
Cicutoxin is not biphasic like the sister toxins of poison hemlock, though the convulsive episodes with alternating relaxation pattern does occur [14]. As stated above, cicutoxin acts exclusively and potently on the central nervous system [11, 15]. Symptoms of cicutoxin poisoning consist of: seizures that often lead to lacerations on the tongue, cheeks, and lips as well as injuries to the head and limbs [12]. During these violent convulsions the head typically arches backwards and the legs stiffen [12]. The eyes, though closed tightly along with the mouth, display dilated pupils [12]. During the periods of relaxation there is an increase in body temperature (hyperthermia) and a chewing or grinding of the teeth [12]. This seizure/relaxation pattern usually ceases with a final paralytic seizure ending in anoxia and death [12]. As in the seizure/relaxation periods with the sister toxins of poison hemlock, each period of relaxation is shorter than the preceding period of relaxation [12]. The only treatment for these seizures is diazepam or phenobarbital and fluid replacement [11-12]. Flashing back to the era known for rampant poisoning, if one of the ladies of the court had decided to use one of the hemlocks, poison hemlock for this scenario, on a targeted enemy, the poisoner would have the added benefit of the poison not taking affect for fifteen to sixty minutes after their victim had ingested the poison depending on the dosage administered. The ‘Lady of Poison’ would have seen her victim first manifest signs of discomfort due the burning sensation of the mouth, throat and stomach at the initial ingestion of the poison. This would have been followed by the victim’s increase in heart rate and breathing. Frothy saliva would begin to appear at the victim’s mouth as would a slight tremor and loss of coordination of the limbs become detectable. The victim would then begin seizing violently before relaxing for a short period of time before seizing again. This pattern would only cease once the victim suffered a final seizure and all muscles in his body began to relax to such an extent that even the victim’s breathing would cease. Once again, though more brutal than tubocurarine poisoning, the outcome would still be the same, a few more years in power for the ‘Lady of Poison.’ A Poison from the Garden Castor Bean Though the above mentioned poisons are extremely potent, it would seem the toxin of choice today is the toxic lectin ricin that is found in the beans of the castor bean plant, Ricinus communis and can be ground into a fine white powder that is easily soluble in water and can remain stable in a wide range of pH's [16]. This particular toxin has found its way onto the Centers for Disease Control and Prevention’s (CDC) bioterrorist list [16-17]. It is classified as a Category B bio-agent and a weapon of mass destruction due to its availability, ease of isolation and production, its stability, lethality, its ability to be rapidly metabolized by the body and can be administered via ingestion, injection or inhalation [17-19]. This toxin only needs two to eight seeds, roughly 500 µg, to be ingested in order to administer a lethal dose [17,19]. The toxin is released when the hard seeds are cracked open during chewing and once released into the body, the resulting protein synthesis inhibition leads to cell death [17]. This toxin is present in all parts of the plant but the seeds contain the highest concentration (1% to 5%) [17,19]. This poison was made famous in 1978, during the Bulgarian Georgi Markov murder case or as some may know it … the “Umbrella Murder” [19]. Though this case may have been the one to make ricin famous, ricin’s infamy did not stop there. There were two cases in 2002, three cases in 2003, and one case each year from 2004 to 2008 where this dangerous toxin was found in the wrong hands [19]. This toxic lectin is composed of a two-part stoichiometry that allows for one heck of a "wham-bamm-thank-ya-ma'am" tag team effort to disrupt the body’s biological functions on a cellular level. Ricin is considered a heterodimeric type-2 ribosome-inactivating protein (RIP – aptly named) [19]. This type 2 configuration holds both an A chain, which serves as the actual ribosome inactivating enzyme, and a B chain that is a galactose/ N-acetylgalactosamine-binding lectin [18-19]. These two chains are covalently linked via a disulfide bond [19-19]. This B chain is inactive by itself but when linked to the A chain it serves as a method of entry into the cytosol of a cell [19]. This cytosol entry is dependent upon hydrogen bonding between the amino acid residues of the B chain and the complex carbohydrates on the surface of eukaryotic cells that have either N-acetyl galactosamine or β-1,4-linked galactose residues on their terminal end [19]. An illustration of the ricin protein is seen in Figure 4. Once this protein enters the cytosol of a eukaryotic cell, it is engulfed by endosomes and hand delivered to the Golgi apparatus [19]. The lysosome and endosomes that would normally digest foreign proteins are harmless to ricin due to its ability to withstand an increased pH range [19]. Once at the Golgi, ricin uses retrograde transportation to travel through the Golgi and gain entrance into the endoplasmic reticulum (ER) [19]. This is where the A chain does its worst. Once inside, the A chain begins to inhibit all protein synthesis in the ER by inactivating all of the present ribosomes by binding a specific adenine ring and depurinating it [19]. This action is irreversible [19]. This ring then gets pushed between two tyrosine rings and is hydrolyzed via N-glycosidase (26). This stops all elongation of any polypeptides which kills the cell [19]. As if this mechanism were not an instant death sentence, ricin infestation can also cause apoptosis, cell membrane damage, function and structure alterations and cause a cytokine storm that would bring about an inflammatory response from nearby cells [19]. Ricin is such a destructive protein that it only takes one single protein entering the cytosol in order to stop 1500 ribosomes per minute from functioning until the cell dies [19]. Symptoms of ricin poisoning depend on the dosage as well as the method of entry. If ricin were inhaled, symptoms would appear within the first eight hours whereas if the toxin were ingested then the first symptoms may display within the first six hours [19]. However, if inhaled, there is a possibility that respiratory symptoms may not manifest for the first 20 to 24 hours after inhalation [19]. Death usually occurs within thirty-six to seventy-two hours after exposure [19].
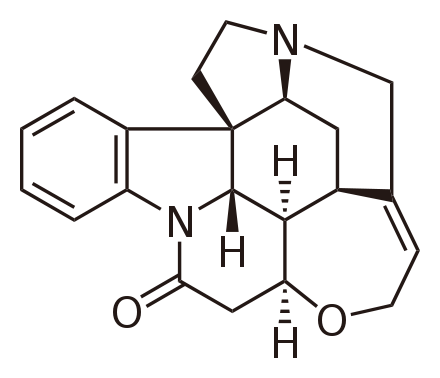
If ricin were ingested, symptoms would consist of vomiting and diarrhea [19]. Both may become bloody and become accompanied by bloody urine as the toxic condition begins to cause gastric bleeding [16, 19]. This would lead to massive dehydration, low blood pressure, hallucinations and seizures [19]. This condition would lead to massive organ failure and result in death [19]. If ricin were inhaled, symptoms would begin with respiratory distress, fever, nonproductive cough, nausea, tightness of chest, heavy sweating, possible fluid retention in the lungs causing the skin to turn blue due to lack of oxygen, low blood pressure and finally respiratory failure or cardiac arrest leading to death [16, 19]. With this route of entry, the potency of the toxin depends on the protein synthesis inhibition, cytokine storm severity, and epithelial membrane injuries [19]. If ricin were injected, as in the "Umbrella Murder", symptoms could be delayed for ten to twelve hours and present with a fever, headache, hypotension, anorexia, abdominal pain and finally followed by multi-organ shut down [19]. Like the previous toxins, there is no medical treatment only symptomatic and supportive treatment. Gastrointestinal suction and activated charcoal administration along with fluid replacement, vasopressor, and electrolyte replenishing therapy are considered the main treatments for ricin poisoning [19]. For treatment of inhaled ricin, oxygen, endotracheal intubation and bronchodilators are suggested [19]. Assuming that the ladies in the 16th to the 18th century were not capable of aerosolizing or injecting ricin, it is assumed that if ricin was chosen to poison an enemy it would be delivered via the oral route. This type of route would be slow acting and the poisoner would have to go on faith that the ricin would have an effect on the victim. Within six hours the victim would start to complain of nausea, diarrhea and vomiting. These symptoms would continue to an extent that all excretions would begin to become bloody. This massive fluid loss would lead to dehydration which would lead to possible hallucinations and low blood pressure. As the victim’s condition worsened, his organs would begin to shut down until shock set in along with seizures culminating in death. Especially Potent Poison Strychnine Strychnine is another alkaloid (see Figure 5) that is similar to the curare plants in its mechanics, but this particular plant is fatal in exceedingly small doses. The toxin is isolated from the seeds of the Strychnos nux-vomica tree of Asian-Indian origin, Strychnos toxifera, a plant of South American origin, Strychnos spinosa, a plant from the tropics of Africa or Strychnos potatorum, a plant of Indian origin [10, 20-22]. For the remainder of this section the focus will be on the Strychnos nux-vomica tree whose seeds contain 1.1% to 1.4% strychnine [21].
Strychnine is a stimulant to the central nervous system (CNS) that causes muscle spasms, convulsions (seizures) that are triggered by the toxins ability to magnify the individual’s sense of touch, olfactory, hearing and sight [10, 21-22]. These heightened sensations are what cause these seizures to often be re-occurring with only a short duration of exhausted relaxation between episodes [10, 21]. In essence, strychnine is a competitive/non-competitive antagonist to glycine for the inhibitory neurotransmitter receptors positioned in the spinal cord and the brain stem [21-22]. This blocking of a natural neurotransmitter inhibitory (glycine) results in an increase in muscular activity due to the unchecked increase in the activity of the neurontransmitters in the spinal cord and brain stem [22]. This increase in muscular activity can lead to: an increase in lactic acid resulting in metabolic acidosis, hyperthermia from the heat generated via constant muscle contraction from the increased motor neuron stimulation or rhabdomyolysis that leads to myoglobinuria that can result in renal failure [22]. The two most detrimental muscles to be affected by this toxin are the respiratory and cardiac muscles. This toxin is often lethal due to its ability to cause spasms of the respiratory muscles leading to respiratory arrest that would then lead to death via asphyxiation. If this toxin acted on the cardiac muscle, an individual would suffer from irregular or rapid heart palpitations followed by cardiac arrest culminating in death [22]. There are three possible mechanisms for this alkaloid. Two of the mechanisms are post-synaptic, but differ in that one mechanism is believed to be competitive while the other is believed to be non-competitive [23-24]. The remaining mechanism, while still seen as a theoretical possibility, displays a non-competitive pre-synaptic binding that seems to contain a trickle-down effect [23]. The first mechanism to be discussed will be the widely studied post-synaptic competitive mechanism. In a normally functioning system, glycine binds to an inhibitory receptor located on motor neurons called Renshaw cell-motor neurons located on the neuron's synapses [20-21]. When this glycine-inhibitory binding occurs, the motor neuron firing necessary for muscle contraction is inhibited allowing for relaxation [21]. In the competitive post-synaptic mechanism of strychnine, strychnine competitively competes against glycine for these receptor sites located on the inhibitory receptor sites on these motor neurons [21]. This action not only stops glycine from binding the inhibitory site, but also decreases the amount of glycine released from these sites which creates a loop ultimately lacking in any inhibitory action [21]. An interesting fact discovered in one study showed that upon the initial appearance of strychnine in the system, there was no change in glycine binding. It was only until there was a specific level of strychnine present that strychnine binding overpowered glycine binding [23]. Once at this specific level of surrounding strychnine, the strychnine out-bound glycine 2 to 1 and only continued to increase in strychnine’s favor as the level of strychnine in the system increased [23]. This may mean that these targeted motor neurotransmitter receptors are actually more sensitive towards strychnine than glycine [23]. This induced lack of the inhibition of motor neuron firing leads to excessive motor neuron activity, inducing convulsions and increased muscular contractions [21]. Another side effect of this neuron overstimulation is that the individual's sense of hearing, sight, touch and smell are also over-sensitized [10, 21]. This heightened sensitization of the senses means that even a slight stimulus to any of these senses has the ability to trigger reoccurring seizures and convulsions [10, 21]. The next mechanism is very similar to the previous one and leads to the same end result; only in this scenario, strychnine performs as a non-competitive inhibitor of glycine inhibitory binding to the motor neurotransmitter synapsis [24-25]. This non-competitive action is due to the belief that strychnine binds to a site that shares a slight overlap with the glycine receptor site, but that in fact two distinct binding sites exist [24-25]. This theory bases itself on the understanding that glycine inhibition acts via a pentameric chloride channel protein that consists of both α and β subunits [24]. These two subunits display a homology to the subunits of both γ-aminobutyric acidA (GABAA) and nicotinic acetylcholine receptors [24]. These three receptors also share a quaternary structure, which is a common aspect among this particular group of neurotransmitter gated ion channels [24]. This same study proved that the α-subunit of the glycine receptor can change formation and become more sensitive towards strychnine instead of glycine leaving these ion channels open for a virtual chloride ion free flow leading to an increase in muscle excitation and contractions [24]. This study proved that under this model, strychnine was three magnitudes higher in binding potency compared to glycine [24]. However, the same study showed that if there was an increase in glycine levels, then increased binding of glycine would occur [24]. This lead to the belief that each receptor may contain more than one binding site leaving glycine and strychnine to compete for the same receptor just utilizing two distinctly and conformationally different, yet overlapping binding sites [24]. Though it is generally accepted that agonists of a particular receptor site induce a larger conformational change in the site over that of the change induced by the antagonist to that site, it would seem that this may not be true in this case since glycine is more readily disassociated from its receptor site as opposed to strychnine, which once bound has a tendency to stay bound to its receptor site [23-24]. This ability of strychnine to stay bound may be due to residual binding of charged amino acids (histidine around the binding sites that underwent conformational changes upon strychnine binding and the tyrosine or cysteine residues associated with the N-terminal region) associated with the receptor site to the charges of the strychnine molecule [24]. All of these amino acids, histidine, tyrosine, tryptophan and cysteine contain α-subunits homologous to the α-subunit of the nicotinic acetylcholine receptor [24]. Arginine residues with a positively charged side chain can also increase strychnine binding via the molecular interaction with strychnine's carbonyl group [24]. The final proposed mechanism of strychnine is a pre-synaptic effect on the actual release of inhibitory transmitters which in turn reduces the inhibitions of the collateral and VIII nerve [23]. This would mean that strychnine would inhibit the release of acetylcholine from the cervical ganglion into the peripheral nervous system, leading to strychnine having an indirect effect on the cholinergic nerve endings which may explain the hypersensitivity aspect of strychnine poisoning due to the increased nerve activity from the lack of inhibitory transmitters released from the ganglion [23]. Strychnine is a colorless, bitter tasting and odorless powder that is readily absorbed via the gastrointestinal tract, respiratory tract and via the skin [21-22]. The symptoms of strychnine poisoning via ingestion usual manifest within ten to twenty minutes post ingestion with cardiac or respiratory arrest occurring thirty five minutes after ingestion [22]. When strychnine is absorbed via the mucous membranes (i.e., intranasal or subcutaneously) symptoms can manifest as soon as five minutes to one hour after exposure to the toxin depending on the dosage. Due to strychnine’s ability to rapidly metabolize (15% of all metabolized strychnine being in the form of strychnine N-oxide) in the body and its seemingly accumulative effect on the body’s inhibitory processes, the toxin is shown to only have an internal half-life of ten to sixteen hours with 20% being excreted via the urine in an unchanged state [21-22]. Treatment for strychnine poisoning is tricky and depends on the route of exposure and the dosage received [21]. Once symptoms begin the individual should be moved to a dark, quiet room if a sensory deprivation chamber is not available [21]. An individual with strychnine poisoning should not be intubated and nasogastric lavage should be avoided due to the sensation possibly triggering a seizure [21]. Induced vomiting should also be avoided due to danger of asphyxiation should an onset of contractions activate during the actual vomiting [21]. Benzodiazepines (i.e., anti-spasmodics) or short acting barbiturates should be administered intravenously to stop or lessen the muscle contractions [20-21]. If the above medications are not sufficient, then heavy sedation or induced paralysis via pancuronium or vecuronium (known neuromuscular blockers) should be induced and the individual intubated at this point [20-21]. Common complications with strychnine poisoning are: respiratory or metabolic acidosis, muscle damage causing myoglobinuria leading to rhabdomyolysis culminating in renal failure [21]. If hyperthermia goes untreated, the individual could sustain anoxic brain damage along with multi-organ failure and disseminated intravascular coagulation [21]. With the above in mind, if one of the devious ladies of the court were to choose this particular toxin to rid the court of an unwanted obstacle, the following would have been the final performance the victim would have performed on the esteemed "Lady of Toxin's" stage: In this scenario, it will be assumed that the route of exposure will be via ingestion since this route is easier to control than inhalation when in a crowded gathering. Within fifteen to thirty minutes the victim would begin to notice his senses of sight, hearing, olfactory and touch becoming more distinct than usual [21]. This increase in sensitivity would soon trigger the first of several clonic convulsions [21]. The victim's muscles would begin to rotate through rapid periods of contractions followed by relaxations giving the victim a "jerking" motion [21]. This convulsion would last for anywhere from thirty seconds to two minutes [21]. Once this initial convulsion ended the victim would only be allowed a moment's peace before the heightened sensations would trigger yet another clonic seizure. These seizures would start to become more tetanic in nature [21]. This means the victim would begin to display outward signs of sustained muscle contractions. These tetanic seizures would cause the victims heels and head to arch backward making the body form a large "bow" formation due to extreme hyperextension, a condition known as opisthotonus (4, 21]. During this time the jaws would become locked shut while the muscles of the face would also contract giving the victim the appearance of a grotesque grin, a condition known as "risus sardonicus" [21]. The eyes of the victim would assume a fixed stare with dilated pupils and can even move around in different directions as they begin to protrude from their sockets [21]. These tetanic seizures affect muscles of the diaphragm along with chest and abdominal muscles leading to respiratory distress causing anoxia and cyanosis [21]. Throughout this entire scenario the victim is fully conscious and in excruciating pain [21]. Once again, following each of these tetanic seizures is a period of complete muscle relaxation, allowing the victim to breathe normally relieving the cyanosis and pupil dilation and allowing the victim to fall into a deep exhausted sleep [21]. These relaxation periods would last for ten to fifteen minutes after which even the slightest stimuli to any of the heightened senses would trigger another tetanic seizure with each convulsive episode being more intense than the previous with a shorter duration of relaxation between each episode [21]. The victim will suffer approximately five of these episodes before expiring of respiratory arrest since very rarely does an individual live past five of these tetanic episodes [21]. Conclusion In conclusion, the de’ Medici era of poisoning was one filled with deceit, dangers, vendettas and fear. No one was considered safe from threats of dying violently. This paper only covered the mechanisms and symptoms of a scant few of the poisons that were running rampant during that time in history. In addition to poisons isolated from plants, there were a considerable number of poisons made from chemicals, heavy metals and even household items (i.e., the ground up mercury glass mirror) that were utilized during that era. Though the origins of the poisons changed, along with the method of delivery, the outcome was almost always the same... death. So next time you go to drink or eat after having left your seat at a dinner party, you may want to think about smelling your food or using that moment when you raise your glass during a toast to check for any "special additives" that may be awaiting your ingestion. References
|