|
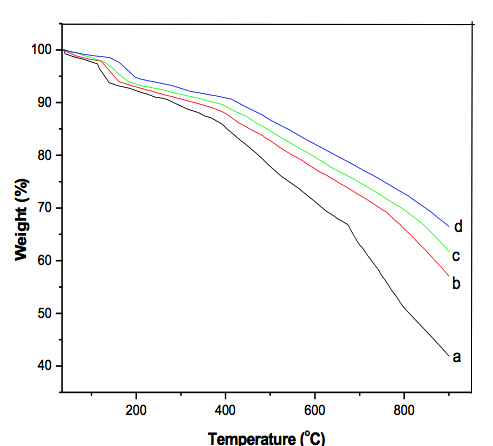
Introduction Conducting polymers are important materials with applications in rechargeable batteries, sensors, electrochemical display devices, and microwave absorbing materials [1, 2]. Research in the field of these polymers has been aimed mainly at some suitable modification of existing polymers so that their performance can be improved. Conducting polymer based nanocomposites possess the advantages of both low dimensional systems (nanostructure filler) and organic conductors (conducting polymer). In recent years conducting polymer based composites containing inorganic oxides or salts of different metal nanoparticles have been of special interest due to their unique electromagnetic properties and their potential applications in several important technological fields [3, 4]. Nanometer sized iron oxide, in the crystalline form of magnetite (Fe3O4) and containing supermagnetic and ferromagnetic properties, has received immense interest because of its numerous applications in various fields, e.g., magnetic recording media, giant magnetoresistive sensors, and photonic crystals [5,6]. Transition metal oxide nanomaterials, such as copper oxide, zinc oxide, and iron oxide, have special physico-chemical properties arising from the quantum size effect and high specific surface area, which can be different from their atomic or bulk counterparts. The large surface to volume ratio of the nanoparticles results in the formation of composites with unusual physical and chemical properties. Compared with organic polymer ferromagnets, conducting polymer–inorganic ferromagnetic composites are considered to be easier to prepare and easier to be put into use. The majority of authors who have worked on the synthesis of conducting polymer/ Fe3O4 composites have reported that the conductivity increases with increasing the loading of fillers [7, 8]. A conducting polypyrrole–ferromagnet composite film has been prepared by means of the technique of anodic oxidation [9]. However, because the quantity of the composite product was limited by the electrochemical method, it is still desirable to synthesize conducting polymer composites with both conducting and ferromagnetic behaviors by a chemical method that can produce larger quantities. Hetero-aromatic organic molecules containing nitrogen and sulphur have very interesting properties. Among this class of polymers, polypyrrole and polythiophene have been studied extensively owing to their good conductivity and relative stability. Polyindole (PIN) is also an electroactive polymer which can be obtained from the electrochemical oxidation of indole or chemical oxidation using different oxidants [10]. However, only a limited number of investigations have been carried out on chemically synthesized PIN [11, 12]. PIN possesses high electrical conductivity; however, it has poor thermal stability and processability, and is insoluble, infusible, and brittle. In order to overcome these problems and obtain a useful magnetic polymer, we developed a simple, inexpensive, and environmentally friendly in-situ polymerization process to synthesize magnetite / polyindole nanocomposites and to analyze for the influence of magnetite nanoparticles in thermal, AC and DC conductivity. Experiment Materials and Methods Indole (Sigma-Aldrich, India), FeCl3. 6H2O, FeCl2. 4H2O, ammonium persulfate (APS), sodium dodecylsulfate (SDS) and ethanol, from Merck, India, were used for the synthesis. Deionized water was used as a solvent for all solutions. Fe3O4 nanoparticles with a particle size of 32 nm were prepared by the chemical co-precipitation techniques as described previously [13]. Synthesis of Polyindole/ Fe3O4 Nanocomposites PIN/ Fe3O4 nanocomposites were synthesized by in-situ polymerization of indole in aqueous solutions containing magnetite nano fluid using ammonium peroxodisulfate as oxidizing agent [14]. Fe3O4 nanoparticles ( 5, 10, 15, and 20 wt %) were mixed with SDS in distilled water and ultrasonicated for 10 minutes, followed by dispersing with the indole (0.5 M) and again ultrasonicated for a period of 20 minutes; ammonium peroxodisulfate was then gradually added into the stirred Fe3O4/indole solution at 100C. The polymerization was carried out at room temperature for 8 hours with constant mechanical stirring. The precipitated PIN/Fe3O4 nanocomposites were filtered and rinsed with distilled water and ethanol. The synthesized polymer composites were vacuum dried at 500C for 24 hours. Polyindole was also synthesized via the same preparation without using the Fe3O4 and SDS. Analytic Methods Thermal stability of the polymer composites was investigated by a Perkin Elmer thermogravimetric analyzer with pure nitrogen gas at a heating rate of 200C/ min. Electrical conductivity of the polymer materials was measured on pressed pellets (circular shape of 0.3 – 0.5 mm thick, 1.2 cm diameter) with the use of a hydraulic press by applying 3 metric ton pressure at room temperature. AC resistivity of the samples was measured with a Hewlett–Packard LCR Meter, fully automatic system in a frequency range 100–106 Hz at room temperature. Dielectric constant or relative permittivity was calculated using the formula: εr = Cd/ εoA where d is the thickness of the sample, C the capacitance, A the area of cross section of the sample, and εo is the permittivity of free space. εr is the relative permittivity of the material which is a dimensionless quantity. From these measurements, ε r and tanδ for the nanocomposites were determined. DC conductivities at different temperatures were measured using a standard four-probe method with a Keithley 2400 system digital electrometer. Results & Discussion Thermal Stability The TGA thermograms of PIN and PIN/Fe3O4 nanocomposites, determined with four different concentrations of nanoparticles, are displayed in Figure 1. All of the samples show three stages of mass loss. The initial mass losses (50 to 1600C) were due to the volatilization of water and oligomers elimination. The second mass loss (200 to 3500C) has been inferred to the decomposition of unreacted monomer and dopant molecules from the polymer [15]. The final mass loss, from 415 to 6800C, is attributed to the further decomposition of carbon containing residues. As shown in Figure 1 (curves b-d), the decomposition temperatures of the nanocomposites were higher than that of PIN and shifted towards higher temperatures as the mass percentage of Fe3O4 increased. This is attributed to the nanoparticles decreasing the rate of the degradation of the polymer and impacting the shape of the TGA curves. PIN showed 50% mass loss at 8000C, while the decomposition losses of the nanocomposite (10 wt %) were about 34% at the same temperature. The improved thermal stability is apparently to a reasonable degree of scientific certainty due to the coordination interaction between Fe3O4 and polyindole chains.
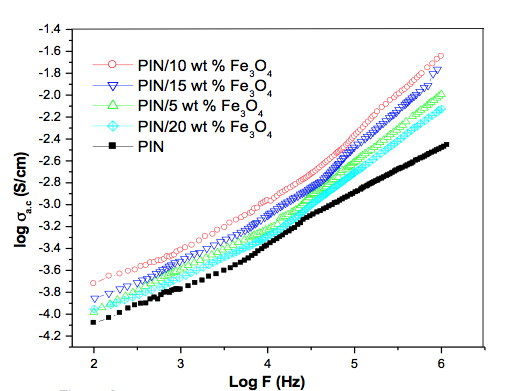
AC Conductivity The variation of AC conductivity of PIN and PIN with various concentration of Fe3O4 nanocomposite is presented as double logarithmic plots of σ AC vs frequency in Figure 2. The AC conductivities of the composites were significantly higher than that of the virgin polymer. The pure, compressed, synthesized PIN was very light with poor linkages, resulting in relatively poor conductivity. The most important, and interesting, observation is that the conductivity was highest for 10 weight percent of Fe3O4 in the polymer composite. In the present study, the composites were synthesized under identical conditions with the monomer indole first adsorbed on the surfaces of the Fe3O4 nanoparticle. Upon addition of oxidant, polymerization took place on the surface of each nanoparticle. The interaction of the metal nanoparticles and polyindole in the composite seems to strengthen the compactness of the original samples. This improves the link between the grains and the coupling through the grain boundaries becomes stronger which ultimately results in increased conductivity in the PIN/Fe3O4 nanocomposites as compared to pristine PIN. The decrease in conductivity with increase in concentration (above 10 wt. %) of nanoparticle is due to the agglomeration of nanoparticles. Moreover, the composites with 20 wt. % sample exhibit a lower AC conductivity than 5 wt. % composite throughout the entire frequency range. The conductivity of nanocomposites depends on the polarity of polymer matrix, surface area, shape of nanoparticles, interfacial interaction between the polymer and nano-filler, and also the quality of conductive network formed [16]. Therefore, at higher concentration of nanoparticles, the interfacial interaction between the polymer and Fe3O4 is poor due to the greater number of metal oxide particles in the polymer matrix.
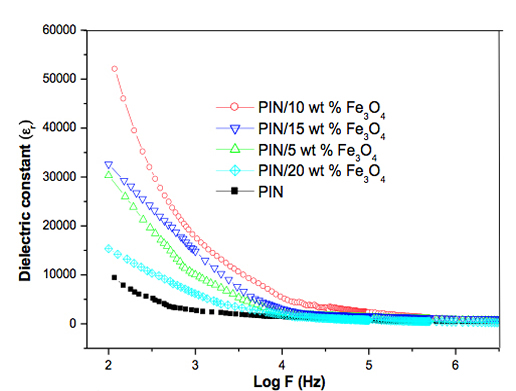
Dielectric Behavior The variation of dielectric constant (εr) and dielectric loss tangent (tan δ) as a function of frequency ranging from 100 – 106Hz at room temperature are presented in Figures 3 and 4, respectively. The dielectric constant initially decreased rapidly with increase in frequency and then, at frequencies higher this 104Hz, became nearly constant. The dielectric properties of materials are mainly analyzed in terms of their polarizabilities at a given frequency. The electron exchange between the Fe atoms of Fe3O4 and nitrogen atoms of PIN, results in local displacement of electrons in the direction of the applied field, which induces interfacial polarization. This type of polarization principally influences the low frequency (102 – 104Hz) dielectric properties. As the frequency decreased, the time available for the drift of charge carriers increased and the observed value of dielectric constant became significantly higher than that of PIN.
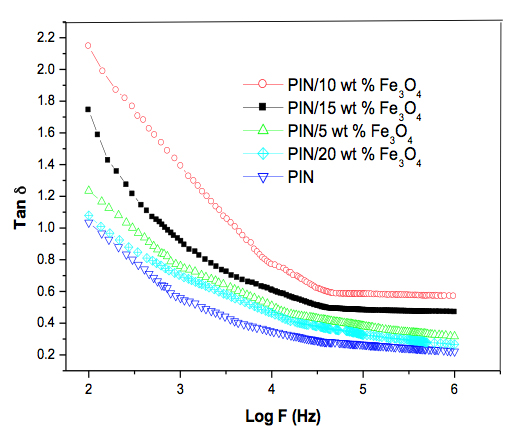
The dielectric constant increased with the increase in concentration of nanoparticles up to a certain concentration (10 wt. %), but the magnitude of conductivity decreased with further addition of magnetite (i.e., 15 to 20 wt. %) (Figure 3). This can be explained on the basis of space charge polarization and reversal of the direction of polarization [17]. At higher loading of fillers, the particle-to-particle distance between the fillers was too short; therefore, the contribution to the dielectric constant by space charge polarization and rotation of the direction of polarization occurring mainly as the interface diminishes. As a result, the magnitude of the dielectric constant was lower at higher content of magnetite nanoparticles. Figure 4 shows that the tan δ also decreased steeply with increasing frequency. At highest frequency the tan δ became constant. At lower frequencies the tan δ values were higher for higher content of magnetite nanoparticles. This is due to the increased interaction between the nanoparticles and polymer chains leading to an ordered arrangement of the composites, which causes a space charge build up at the interfaces [18]. This accumulation of space charge leads to an increase in dielectric loss due to the movement of virtual charges that get trapped at the interface of a multi-component material with different conductivities. The dielectric loss tangent value of PIN was lower than the composites and among the composite, 10 wt. % of sample exhibited the higher dielectric values. The higher surface area of nanoparticles provide better particle to particle contact and this leads to a higher packing of chain inside the polymer composite. It was responsible for the higher tangent values. Further, the sudden decrease in dielectric constant above 10 wt. % of composite was due to the agglomeration of nanoparticles in the polymer chain. This reduces the segmental mobility and hence increases the rigidity of the chain. The improvement in electric properties suggests that the fabricated PIN/Fe3O4 nanocomposite has potential application in the fields of nanotechnology, plus multifunctional material in various electronic industries.
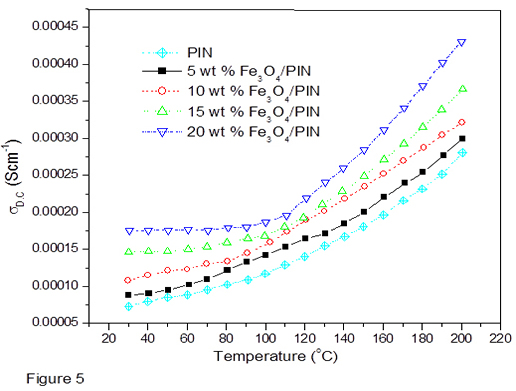
Temperature Dependent DC Conductivity Studies The temperature dependence of the electrical conductivity of PIN and four types PIN/Fe3O4 composites at various temperatures from 300C to 2000C are given in Figure 5. Two ranges of near linear variations with applied voltage were obtained for the Fe3O4 nanoparticles incorporated PIN. In the case of the nanocomposites, the growing polymer chains were presumed uniformly adsorbed on the surface of the magnetite particles and, thereby, led to an arrangement of nanoparticles in the polymer matrix.
From the figure, it can be noted that from 300C to 1000C, the conductivity values did not show much variation (>10 wt. % of nanoparticles) and increased suddenly in the temperature range from 1000C to 2000C. At low temperature, the polymer can behave like a hard and glassy material. PIN has a glass transition temperature (Tg) of 1280C and a melting temperature (Tm) of 2730C. The higher conductivity of PIN/Fe3O4 composite at higher temperatures is due to the phase transition of polymer composite from the glassy state to a rubbery region [11]. Moreover, it is important to mention here that the high value of DC conductivity above Tg is apparently attributed to the increase in the number of conduction paths created by the interaction between magnetite nanoparticles and the polymer chains. Conclusions Polyindole with different mass percentage of Fe3O4 (0, 5, 10, 15, and 20 wt. %) was prepared by an in situ polymerization method. TGA results indicated that the PIN/Fe3O4 composite had better thermal stability than that of PIN and the thermal stability of the composite increased with an increase in concentration of metal oxide nanoparticles. This might be due to the interaction between the electronegative nitrogen of PIN and the magnetite nanoparticles. AC electrical conductivity, dielectric constant, and dielectric loss factor of PIN/Fe3O4 were studied as a function of frequency at different volume fractions of nanoparticles. The electrical properties of the composite increased with an increase in concentration of magnetite up to 10 wt.% and with further addition of nano-fillers, the conductivity was found to decrease. The higher value of conductivity of the composite was apparently due to the increased polymer filler interaction. The dielectric constant and loss factor increased with an increase in Fe3O4 weight percentage in the composite up to a certain concentration (10 wt. %) of filler. These properties (dielectric constant and loss factor) decreased with further addition of nanoparticles. The high values of the dielectric constant of the nanocomposite suggest a possible application of these materials in the field of actuators and sensors. DC conductivity of the polymer composites was higher than that of the PIN and the conductivity increased with an increase in mass percentage of magnetite nanoparticles. Acknowledgement The authors wish to thanks Prof. K. R. Haridas, School of Chemical Science, Kannur University for providing TGA facilities in the department. References
|