
|
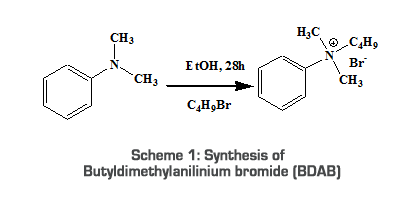
Introduction Phase transfer catalysis has emerged as a useful green chemistry technique for carrying out organic synthesis with ease and economical viability. Phase transfer catalysts help the reactants in different phases to come together and react with each other, often with catalytic efficiency under milder conditions that can be easily devised. Since its discovery, phase transfer catalysis has been in use for more than three decades and is an established technique nowadays in organic synthesis [1]. In a heterogeneous system of two immiscible solvents, reaction between the reactants contained in them is very slow due to the lack of effective interaction. This problem can be solved using organic solvents such as ethanol, dioxane, acetone, etc. But the difficulty has been that most of the inorganic salts are less soluble in these solvents; moreover, some of these solvents are toxic. The problem was remedied, at least in part by the utilization of dipolar aprotic solvents like dimethylsulfoxide, dimethylformaamide, or hexaethylphosphorictriamide. These solvents are toxic, expensive, and are difficult to remove after the reaction. These problems can easily be solved in many cases by the use of the technique of Phase Transfer Catalysis (PTC). This technique was aptly used by Starks [2], and within a short period of time it became an active subject of research with deep implications, especially in preparative organic, organometallic, and polymer chemistry. Phase transfer catalysts are substances which transfer a reactant, from the aqueous phase where the inorganic reactants are normally soluble, across the inter-phase boundary in a two-phase heterogeneous aqueous–organic solvent system, and the phenomenon continues so as to affect the progress of the reaction. These catalysts are used in very small amounts and can perform the important function of transporting the reactant repeatedly into the appropriate phase. A large number of structurally different phase transfer catalysts are currently available. Some of the common catalysts include quaternary ammonium and phosphonium compounds [3], crown ethers [4] and linear ethers [5]. The main class of phase transfer catalysts is crown ethers [6]. These are expensive and toxic in nature. The compounds consisting of quaternary ammonium, phosphonium, arsonium, etc. have also been found to be effective as phase transfer catalysts. These compounds must satisfy at least two fundamental conditions in order to function as phase transfer catalysts (i.e., their solubility in organic phase and absence of steric hindrance around the cationic center to function as an effective cation–anion pair). The types of quaternary ions found to be most effective are those with four relatively large alkyl or aryl substituents on nitrogen, rather than with one particularly long alkyl or aryl chain [7]. A PTC works by encapsulating the ion. The PTC–ion system has a hydrophilic interior containing the ion and a hydrophobic exterior. This paper mainly highlights the synthesis of quaternary ammonium ion with starburst substituents. Starburst compounds are star-shaped, high molecular weight compounds. Quaternary ammonium salts having a structure similar to starburst compounds have already been reported to show excellent catalytic activity [8, 9]. Because of this we have quaternarised a starburst tertiary amine that is then converted to a quaternary ammonium salt, which may act as a good phase transfer catalyst. Also, the starburst tertiary amine compound that we synthesized showed properties such as high organophilicity, large lipophilicity and high electron capturing capacity, which are the most important criteria for a phase transfer catalyst. These substituents are decidedly organic and therefore likely to be soluble in non-polar organic solvents, despite the presence of the positively-charged nitrogen and negatively-charged counter ion. At the same time, the ionic nature of the ammonium ion renders them soluble in aqueous media. This makes them move back and forth between the two phases [10]. By using a phase transfer catalytic process, one can achieve faster reactions, obtain higher conversions or yields, make fewer byproducts, eliminate the need for expensive or dangerous solvents which dissolve all the reactant in a single phase, eliminate the need for expensive raw materials and minimize waste problems. Phase transfer catalysts are especially useful in green chemistry by allowing the use of water and reducing the need for organic solvents [11-13]. The objective of this paper is also to compare the catalytic effects of a known PTC with two synthesized ones, viz; Tetrabutylammonium bromide (known) with Butyldimethylanilinium bromide and 3,5-bis[(2-methyl-naphthylene-1-yl)-phenylamino-phenyl]-butyl-(2-methoxy-naphthalene-1-yl)-phenylammoniumbromide (synthesized) using certain organic reactions. Experimental 2.1. MATERIALS The reagents [2-Naphthol, aniline, N,N-dimethylaniline, Mohr’s salt (Merck, India), 1-Bromobutane, CuCl, Bromobenzene, (Loba Chemie, India), 2-Methoxynaphthalene, Tetrabutylammoniumbromide (TBAB) (SRL India), K2CO3 (NICE chemicals), KI (Qualigens, India)] are purified before use as per common laboratory procedure [14,15]. Silica Gel (60-120 mesh, SRL, India) and Bromine (Merck) are used as such. The solvents were distilled before use according to procedures available in literature [14,15]. Spectroscopic grade solvents (Merck, India) were used for UV-Vis analysis. Melting points were determined in open capillaries using melting point apparatus (JSGW, Gujarat) and are uncorrected. FT-IR spectra were recorded on a Schmidzu 8400 S; UV-Visible spectra were recorded on a UV-Vis. Schmidzu 1700 using 1cm length quartz tube; 1H NMR spectra were recorded on a NMR-JEOL GSX-400 with CDCl3 as solvent. 2.2. METHODS 2.2.1. Preparation of Butyldimethylanilinium bromide (BDAB) N,N-dimethylaniline (0.014mol, 1.76ml) in dry ethanol (25ml) was mixed with 1-bromobutane (0.02mol, 2.17ml). The contents were refluxed for 28 hours with constant stirring. The completion of the reaction was checked by TLC. The solvent was distilled under vacuum and the oily residue was purified using column chromatography. The crude product, BDAB, was washed with ether and allowed to dry. A dark blue, oily liquid was obtained [16].
2.2.2. Synthesis of 3, 5-bis [(2-methyl-naphthylene-1-yl)-phenylamino-phenyl]-butyl-(2-methoxy-naphthalene-1-yl)-phenylammoniumbromide 2.2.2.1. Synthesis of Methoxy naphthyl amine 5ml Conc. H2SO4 was added drop wise to 2-methoxy naphthalene (0.01mol, 1.58g) at such a rate that the temperature does not exceed 5oC. To this, 4 ml Conc. HNO3 was added drop wise with stirring at 0oC. The mixture was stirred at 0oC for an hour, at room temperature and at 55oC for another hour, filtered and dried. It was then refluxed with Mohr’s salt (1.5g) for one hour. The contents were allowed to cool and then filtered and washed with ethanol [17].
2.2.2.2. Synthesis of N,N,N’-Tris-(1-methoxynaphthalen-2-yl)- 1-Amino-2-methoxy naphthalene (0.03mol, 5.67g), tribromobenzene (0.01mol, 3.30g), CuCl (200mg), K2CO3 (1.0g) and KI (1.0g) were refluxed in acetone (20ml) for 10 hours at 60oC. After the completion of the reaction (checked by TLC), it was extracted using ether. Solvent was removed by vacuum distillation and the product was recrystallised from ethanol [18]. Yield: 40.5%; Melting point: 50oC; 2.2.2.3. Synthesis of N,N,N`-Tris-(2-methoxy-naphthalenen-1-yl)-N,N,N’-triphenylbenzene-1,3,5-triamine N,N,N`-Tris-(2-methoxy-naphthalenen-1-yl)-N,N,N`-triphenylbenzene-1,3,5-triamine (4.2g, 0.0071mol) and bromobenzene (0.0213mol, 2.2ml) are coupled using Copper (200mg) catalyst in basic medium by refluxing the contents for 10hour at 60oC [19].
2.2.2.4. Synthesis of 3, 5-bis[(2-methyl-naphthylene-1-yl)-phenylamino-phenyl]-butyl-(2-methoxy-naphthalene-1-yl)-phenylammonium bromide (BPBPB) The compound was synthesized as per the same procedure adopted for the synthesis of BDAB [16].
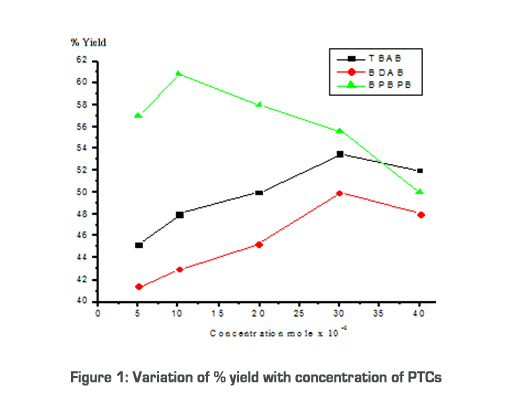
Results & Discussion Quaternary ammonium compounds are widely used industrially and commercially [20]. Owing to their extensive use, exact and simple conditions for their preparation should be summarized. Two types of phase transfer catalysts are synthesized as given in the experimental part and their activity is compared with a commercially available catalyst. Catalytic activity of TBAB, BDAB and BPBPB were tested for the substitution reaction between sodium phenolate and N-butyl bromide. Typical experiments were conducted by mixing 0.03mol of n-butyl bromide in toluene (25ml) and 0.03mol of sodium phenolate in water (20ml) and a suitable quantity of PTCs (0.003mol) at 70oC for 4.0 hours [21]. PTCs are usually used in heterogeneous immiscible liquid phases that are in contact with an aqueous phase containing an ionic reactant and an organic phase containing the organic substrate. Normally the reaction of two substances in separate phases is inhibited because of the inability of the reagent to come together. Adding a PTC solves this problem by transferring ionic reactant into the organic phases. Because the reaction medium is aprotic, an SN2 reaction occurs rapidly [6]. Formation of phenyl butyl ether using PTC follows the below mechanism. 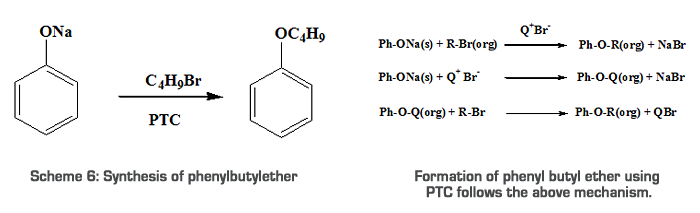 Q+Br- is the PTC, where Q+ (Quaternary ammonium ion) is the lipophilic center, which can move back and forth between the two phases. As the ammonium ion moves from the aqueous phase into the organic phase, it carries with it negatively charged ‘PhO-’ ion. If it travels as the part of an ammonium ion pair, the phenoxide ion can be transported from the aqueous phase into the organic phase in which it is ordinarily insoluble. The phenoxide ion is greatly stabilized through solvation in the polar environment in which it is dissolved. When it is transported into the organic layer, it arrives bare -- shorn of its solvating and stabilizing water molecules. Therefore, it is in a highly reactive state. Unsolvated phenoxide is far more reactive than solvated phenoxide. Under these conditions, formation of phenylbutylether is quantitative and complete in 4 hours. We use three PTCs to carry out the reactions at different conditions (i.e., by changing the concentration of the catalyst). The results are given in Table 1 and shown in Figure 1. From the Table, it can be concluded that BPBPB gives the maximum yield at an optimum concentration of 0.001mol. The reactivity sequence of the three PTCs is as follows: BPBPB > TBAB > BDAB. The different reactivity for catalysts is due to the lipophilic property of cation group in catalyst which governs the formation of catalytic intermediate.
Conclusions Of the three PTCs, BPBPB is efficient for the formation of Phenylbutylether. It is observed that BDAB and starburst PTC are regenerated up to 95% by weight, but TBAB is not regenerated. The catalytic activity of starburst PTC (BPBPB) is almost similar to that of heterogeneous PTC [21, 22]. (See Table 1 for concentration and yield comparison.) The efficiency of starburst PTC is due to the following factors: 1) high molecular weight of aryl group and large organophilicity, 2) lipophilic property of cation group in catalyst due to the presence of same type of aryl group on nitrogen [23]. Due to the above factors, starburst PTC is efficient not only for esterification, but also for many other organic reactions. This PTC is having a wide range of industrial application. Further studies are being conducted on the synthesis of various types of starburst PTC and their utilization in various other organic reactions. Nomenclature
 References
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Boiling point:
Boiling point: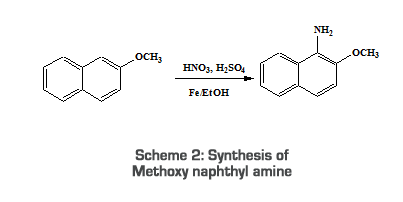 Yield 75.1%; Melting point: 72oC;
Yield 75.1%; Melting point: 72oC;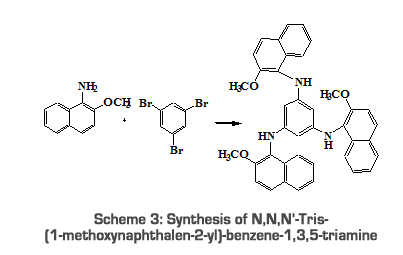
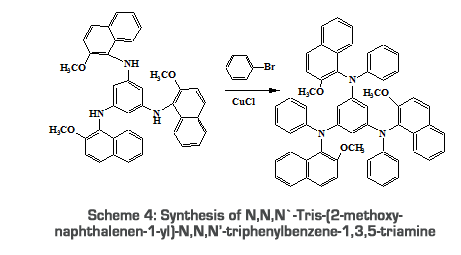 Yield: 38.5%; Melting point: 47oC;
Yield: 38.5%; Melting point: 47oC;![Scheme 5: Synthesis of 3, 5-bis[(2-methyl-naphthylene-1-yl)-phenylamino-phenyl]-butyl-(2-methoxy-naphthalene-1-yl)-phenylammonium bromide (BPBPB)](Images/graph7.png) Appearance: Yellow solid;
Appearance: Yellow solid;