|
Introduction Gastrointestinal (GI) cancer is an important medical problem. The American Cancer Society estimates that during 2006 there were 263,060 new cases and 136,180 deaths due to all GI cancers in the USA. These figures include new cases (22,280; 33,730) and deaths (11,430; 32,300) due to gastric and pancreatic cancer respectively (1). Similarly, the World Health Organization (2) indicates that gastric cancer is the fourth most prevalent cancer globally and the most prevalent cancer in less developed nations while pancreatic cancer ranks only ninth (2) globally; it has a five year survival rate of less than 10% (3), making it a deadly disease. The late diagnosis of pancreatic cancer contributes substantially to its poor prognosis and low survival rate. Hence there is a real need for a minimally invasive early diagnostic method (3). Traditional methods of gastrointestinal cancer diagnosis have included guaiac tests for occult blood, biopsy, exfoliative cytology, endoscopy, barium X-rays, ultrasonography, computer tomography (CAT scans), and magnetic resonance imaging (MRI). Ultrasonography, CAT scans and MRIs, taken in combination with the clinical presentation, have proven the most valuable for the diagnosis of pancreatic cancer (3-4). Additionally, serum tumor antigens have been used as a diagnostic aid to measure tumor burden, and to detect recurrent disease and monitor therapy for pancreatic and other gastrointestinal cancers (5-6). Tumor antigens that have proven useful for the detection of a variety of gastrointestinal cancers include among others: CEA, CA 19-9, CA 72-4, CA 50, CA 195, and CA242. The principal tumor marker in current use for the diagnosis and monitoring of pancreatic cancer is CA 19-9. Likewise, CA 72-4 and CEA are the major tumor antigens associated with gastric cancer and colorectal cancer, respectively (5). Elevated alpha-fetoprotein (AFP) has been extensively used as a marker for hepatic disease, including hepatoma, and for yolk sac-derived germ cell tumors. It has also been reported in a few cases of other gastrointestinal cancers (5-7). CA125 is a marker of ovarian cancer, but has been reported to have some sensitivity for gastrointestinal cancer (5, 8). Elevated CA 15-3 has been reported in a variety of adenocarcinomas, including breast, lung, ovary, colon, and pancreas. It is principally used in the assessment of breast cancer patients (9). CA27.29 is used as a marker for therapeutic monitoring in breast cancer patients (10-11). It has also been reported in some cases of ovarian, uterine, lung, prostate, colorectal, and pancreatic cancer (12). Cyfra 21-1 is used as a marker of lung cancer and has not been reported to be useful in diagnosis and monitoring of gastrointestinal cancer (5, 13). There are reports of elevated serum ferritin levels in patients with hematological cancers, hepatocellular carcinoma, and cancer of the esophagus, pancreas, colon, breast, lungs, and ovaries. (5, 14). Neuron specific enolase (NSE) is used as a marker for small cell lung carcinoma, neuroblastoma and some renal tumors. It has also been reported to be elevated in colorectal and gastric cancers as well as endocrine pancreatic tumors, oatcell cancer, seminoma, melanoma, and medullary thyroid cancer (5) and pheochromocytoma and carcinoid tumors (14). CEA is a 150-300 kDa cell surface heterogeneous glycoprotein which is structurally similar to IgG. Abnormally elevated serum levels have been reported in patients with colorectal cancer, breast cancer, and a variety of other carcinomas (15-16). Additionally, CEA levels can be elevated in heavy smokers and patients with nonmalignant pathologies (17). Consequently, CEA is currently used in therapeutic monitoring and as a diagnostic aid, but is not useful in screening for cancer. It has long been considered to be the “gold standard” for the detection of gastrointestinal diseases. CA 19-9 is a high molecular weight (200-1000 kDa) mucin like glycoprotein which exists as a ganglioside on tumor cells. The expression of this sialylated Lea blood group antigen (sialylated lacto-N-fucopentoeose II ganglioside) is required for the expression of CA 19-9 and hence, Lea-b- patients do not express the antigen and can present as false negatives (18). A monoclonal antibody was developed against CA 19-9 derived from the SW-1116 human colon carcinoma cell line (19). CA 19-9 is clinically useful in the detection of pancreatic, colorectal, hepatic, and other gastrointestinal cancers. It has also been described in breast and lung cancer (5). CA 50 is related to CA 19-9, but lacks a fucose residue. Its epitope is the same as that found in Lea-b- (Lewis negative) patients. It has been reported in patients with gastric, colon, and hepatic cancer (20). CA 195 is also related to CA 19-9. It is defined by the mouse monoclonal antibody CC3C-195 and it recognizes both Lea and sialyl-Lea epitopes. Binding with higher affinity to the sialylated Lea blood group antigen, the antibody can bind to both the sialylated and unsialylated Lea blood group. CA 195 has been reported in pancreatic, colon, and gastric cancers (5). CA 242 is also related to CA 19-9 and CA 50 (21). A mouse monoclonal antibody (CA242) directed at COLO 205 (a human colorectal cancer cell line) and a second antibody directed against sialylated Lewis A detect this antigen (14). CA 242 has been reported in pancreatic, (22), colorectal (23), gastric and liver cancers (21). CA 72-4 is a 1 million kDa mucin-like glycoprotein complex (TAG 72), which is predominantly associated with human adenocarcinoma of the gastrointestinal tract (24-25). Two monoclonal antibodies (cc49 and B72.3) have been developed against it which detect distinct antigenic determinants expressed on the circulating antigen found in a variety of gastrointestinal cancers and lung cancer (26-27). Its use is recommended in cases of gastric cancer and it has been used in tumor panels (ratio of CA 19-9 to CA 72.4) to exclude pancreatic disease (5). CA 125 is a 200 kDa glycoprotein expressed by tissue of mullerian duct origin as well as by ovarian tumors. It is defined by the mouse monoclonal antibody OC 125 derived from an ovarian cancer cell line (OVCA 433). It is currently used for detecting epithelial tumors of the ovary. However, it has also been reported in breast, lung, endometrial, and gastrointestinal tumors. It can be elevated with pregnancy and with pelvic inflammatory disease (28). CA 15-3 is a 300-450 kDa glycoprotein defined by two monoclonal antibodies. The 115D8 antibody recognizes human milk fat globule membranes and the DF3 antibody reacts with a breast cancer antigen extract (29-30). It is principally used to monitor breast cancer patients, but has been reported in cases of ovarian, pancreatic, lung, and colorectal cancer (5). CA 27.29 is a mucin antigen defined by the monoclonal antibody B27.29. This antibody recognizes an antigen extracted from ascites fluid derived from patients with breast cancer. CA 27.29 has an epitope that is shared with the DF3 antibody of CA15-3 (31). It is currently being marketed as a specific test for breast cancer, however it has been reported in some cases of ovarian, uterine, lung, prostate, colorectal, and pancreatic cancer (32). Alpha-fetoprotein (AFP) is a 70,000 kDa glycoprotein which has been isolated from patients with hepatocellular carcinomas and germ cell tumors (33). Maternal serum and amniotic fluid AFP levels are routinely used for the prenatal diagnosis of open neural tube disease and gastroschisis, and together with karyotyping have been used to diagnose cases of Down’s Syndrome (34-35). Alpha-fetoprotein has been reported to be useful in screening for hepatocellular carcinoma in high incidence areas such as Asia, and for classifying and staging germ cell tumors (33). AFP has been reported in hepatocellular carcinoma, testicular and ovarian germ cell tumors, as well as pancreatic, colorectal and gastric carcinomas (7). Ferritin is a 460 kDa intracellular apoprotein that when saturated with iron forms a storage protein of approximately 900 kDa. (36-37). Serum ferritin levels reflect the total iron stores of the patient (37). Increased serum ferritin is also observed in hepatocellular carcinoma (14), acute myelocytic leukemia, Hodgkin’s lymphoma, neuroblastoma, teratoblastoma and cancers of the colon, esophagus, breast, lungs, and ovaries (5). Cyfra 21-1 is a 40 kDa fragment derived from cytokeratin 19. One subgroup of intermediate filament proteins, cytokeratins are found in epithelial cells. The monoclonal antibody recognizes an epitope on the Cyfra 21-1 fragment and is useful in the detection of non-small cell lung cancer, including squamous cell carcinoma of the lung (38). It has also been reported in patients with cervical cancer and other malignancies (39-40). Neuron specific enolase (NSE) is a 78 kDa glycolytic isoenzyme (14). Elevated serum NSE levels have been observed in cancers of neuroendocrine origin. These include small cell lung cancer (SCLS), neuroblastoma, pheochromocytoma, melanoma, medullary thyroid cancer, intestinal carcinoids, and pancreatic endocrine tumors. (31, 5). It is primarily used in the assessment of SCLC (14). The purpose of this study was to compare the analytical and clinical performances of thirteen serologic tumor marker tests (CEA, CA 19-9, CA 195, CA 50, CA 242, ferritin, CA 72-4, CA 125, CA15-3, CA 27.29, AFP, Cyfra 21-1, and NSE) for the detection of pancreatic cancer, gastric cancer, and other gastrointestinal cancers. Materials & Methods ASSAYS All assays were performed according to the directions supplied by the manufacturers. The Tandem®-E CEA assay (Hybritech, Inc) is a solid phase two-site immunoenzymometric assay utilizing two monoclonal IgG antibodies directed against unique sites on the CEA antigen. This assay was quantitated spectrophotometrically using the Photon Immunoassay AnalyzerTM from Hybritech, Inc. The Centocor® CA 19-9TM assay (Fujirebio Diagnostics, Inc./Centocor, Inc.) is a solid phase radioimmunoassay (CA 19-9) using the 1116-NS-19-9 antibody for both the capture and tracer antibodies. This antibody is directed against an epitope, which is biochemically related to the Lewis A determinant; the assay was quantitated using a GenesysTM 5000 gamma counter (Laboratory Technologies, Inc.). The Tandem®- CA 195/Hybri C MarkTM assay (Hybritch Europe, Inc.) is a solid phase two-site immunoradiometric assay (CA 195) utilizing monoclonal IgM antibodies developed against the Lewis A (blood group determinant) and sialyated Lewis A epitopes on the CA 195 antigen. This assay was measured using a GenesysTM 5000 gamma counter (Laboratory Technologies, Inc.). The RIA-gnost® CA-50 assay (CIS bio international) is a solid phase two-site immunoradiometric assay (CA 50) utilizing monoclonal mouse antibodies directed at two carbohydrate chains (sialylated Lewis A and sialylated lactotetraose) of the adenocarcinoma cell line Colo 205. The assay was measured using a GenesysTM 5000 gamma counter (Laboratory Technologies, Inc.). The Diagnostic Automation® CA242 assay (Diagnostic Automation, Inc) is a solid phase enzyme linked immunosorbent assay (CA 242) based on an antibody (C242) directed against a colorectal carcinoma cell line (COLO 205) and another antibody directed against sialylated Lewis A. The assay results were quantitated using the Bio-Tek EL 800 microtiter plate reader (Bio-Tek, Inc). The Centocor® CA 72-4TM assay (Fujirebio Diagnostics, Inc./Centocor, Inc.) is a solid phase radioimmunoassay (CA 72-4) based on two monoclonal antibodies, cc49 and B72.3, which react with distinct antigenic determinants on a tumor associated glycoprotein TAG 72. The antigen was quantitated using the GenesysTM 5000 gamma counter (Laboratory Technologies, Inc.). The Centocor® CA 125TM assay (Fujirebio Diagnostics, Inc./Centocor, Inc.) is a solid phase two-site immunoradiometric assay (CA 125) using two mouse monoclonal antibodies, OC125 directed against the OVCA 433 ovarian cancer cell line and a second antibody directed against another CA 125 epitope. The assay was measured using a GenesysTM 5000 gamma counter (Laboratory Technologies, Inc.). The Centocor® CA 15-3® assay (Fujirebio Diagnostics, Inc./Centocor, Inc.) is a solid phase radioimmunoassay using the 115D8 murine monoclonal antibody as the capture antibody and the I125 labeled DF3 murine monoclonal antibody as the tracer. This assay was quantitated using an Iso Data® gamma counter. The Truquant® BRTM assay (Fujirebio Diagnostics, Inc./Centocor, Inc) is a solid phase competitive inhibition radioimmunoassay using polystyrene tubes coated with CA 27.29 antigen and I125 labeled murine monoclonal B27.29 antibody. This assay was quantitated using an Iso Data® gamma counter. The IMx® AFP assay (Abbott Laboratories, Inc.) is a microparticle enzyme immunoassay (MEIA) utilizing two monoclonal antibodies directed against unique sites on the AFP antigen. This assay was quantitated using the IMx® Automated Analyzer from Abbott Laboratories, Inc. The Diagnostic Automation® Ferritin assay (Diagnostic Automation, Inc) is a solid phase enzyme linked immunosorbent (ferritin) assay using two mouse monoclonal antibodies directed at different sites on the protein. This assay was quantitated using the Beckman CoulterTM AD340 microtiter plate reader (Beckman Coulter, Inc.). The Diagnostic Automation® Neuron Specific Enolase (NSE) assay (Diagnostic Automation, Inc) is a solid phase enzyme linked immunosorbent assay which uses two mouse monoclonal antibodies directed at different epitopes of the gamma (γ) subunit of the NSE isoenzyme. This assay was quantitated using the Beckman CoulterTM AD340 microtiter plate reader (Beckman Coulter, Inc). The Centocor® CyfraTM 21-1 assay (Fujirebio Diagnostics, Inc./Centocor, Inc.) is a solid phase immunoradiometric assay utilizing two mouse monoclonal antibodies, KS19.1 and BM19.21, to detect cytokeratin 19 fragments in serum. The assay was quantitated using a GenesysTM 5000 gamma counter (Laboratory Technologies, Inc.) Statistical analysis was performed using SPSS software. PATIENTS AND CONTROLS Procedures used in this study were in accord with ethical standards established by the University of Southern Mississippi (USM). Permission for the study was granted by the USM Human Subjects Protection Review Committee (HSPRC/IRB) and the hospital IRB. All documents relating to the patients, including informed consent, were maintained by the hospital. Patient samples were given a numerical code and patient names were not divulged to the researchers. All study participants were selected from patients seen in an area hospital. This hospital has a large oncology division. Five hundred and fifty four patients were randomly chosen and the assays were run in a blind fashion. Blood samples were collected using appropriate aseptic technique. Following serum separation aliquots were coded and frozen at -20oC. Subsequently, aliquots were thawed at 37oC and assayed in duplicate (sample permitting) for the tumor antigens. The diagnoses were obtained from the attending physicians and were based on pathological examination. Patient classifications included (a) no known disease, (b) nonmalignant disease, (c) cancer of non-gastrointestinal origin, and (d) specific gastrointestinal cancers. Cancer patients were classified according to the primary site of the tumor, regardless of the presence or absence of metastases. The normal control subjects were healthy males (~100) and females (~100) ranging from 18-65 years of age. Their blood samples were collected and processed in the same manner as the patient samples. Results PRECISION AND LINEARITY Quality control samples were used to determine intra- and inter-assay precision. The within-run coefficient of variation (%CV) was 11% for all but the CA 15-3 (20%) and ferritin (50%) assays which were higher (Table 1). Similarly the between-run coefficient of variation was equal to or less than 16% for all of the assays except ferritin ((41%)(Table 2). Serial dilutions of abnormal pool samples exhibited good linearity with R2 values (CEA [0.99], CA 19-9 [0.99], CA 195 [0.99], CA 50 [0.99], CA 242 [0.98], CA 72-4 [0.99], ferritin [0.97], CA 125 [0.99], CA 15-3 [0.99], CA 27.29 [0.99], AFP [0.99], NSE [0.88], Cyfra 21-1 [0.99]) equal to or greater than 0.97 for all the assays except NSE (0.88).
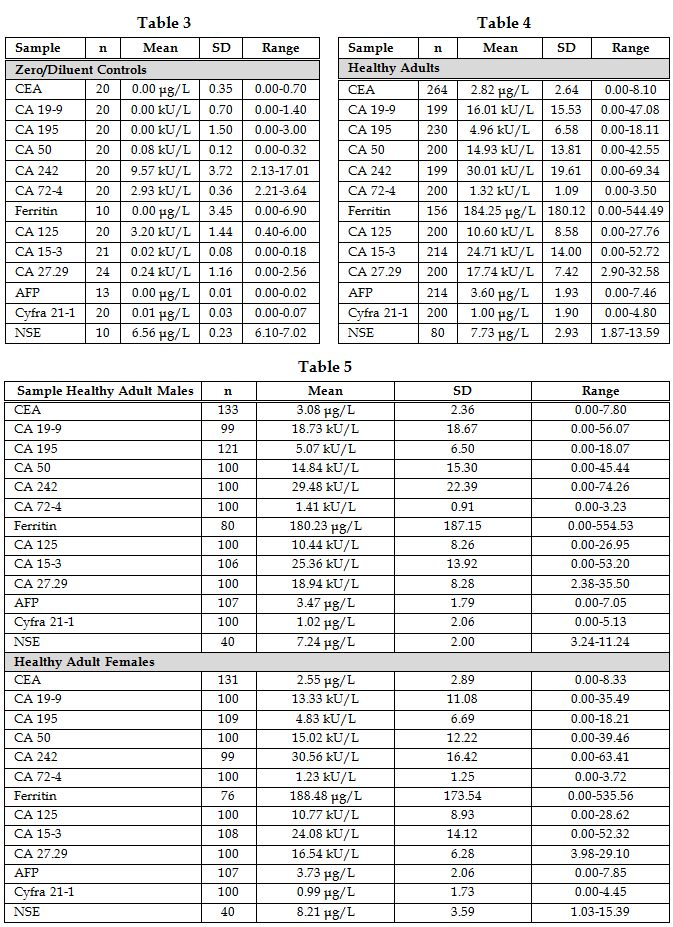
Rerefence Intervals The minimum detectable concentration of analyte (analytical sensitivity) was determined by analyzing approximately 20 replicates of the zero calibrator/diluent, calculating the mean plus two standard deviations, and establishing this as the cut-off value (Table 3). Values falling below this cutoff were presumed to be analyte free. The cutoff for CA 125 (6.0 kU/L [U/mL]), ferritin (7 μg/L [ng/mL]), NSE (7 μg/L [ng/mL]) and CA242 (17 kU/L [U/mL]) were higher than expected. Values for the other assays were equal to or less than 3.6 kU/L [U/mL] (Table 6). The normal adult reference intervals were established by determining the 95% confidence intervals for healthy control male and female subjects. The intervals (Tables 4, 5) were broader than those reported by the manufacturer for all but the CA 125, CA 72-4, CA 27.29, AFP, and NSE assays, which were somewhat narrower. There was no significant difference between healthy adult males and females for any of the assays except CA 19-9, where the males were significantly (p<0.05) higher.
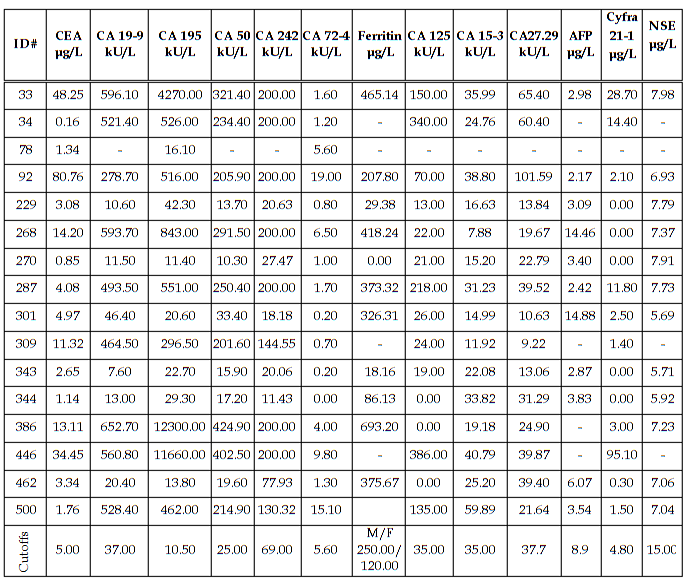
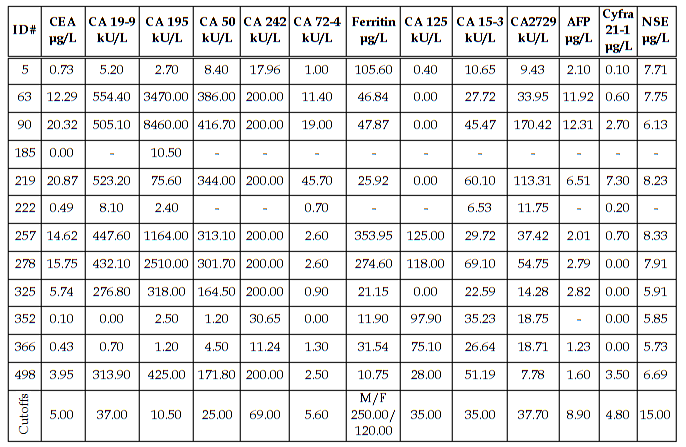
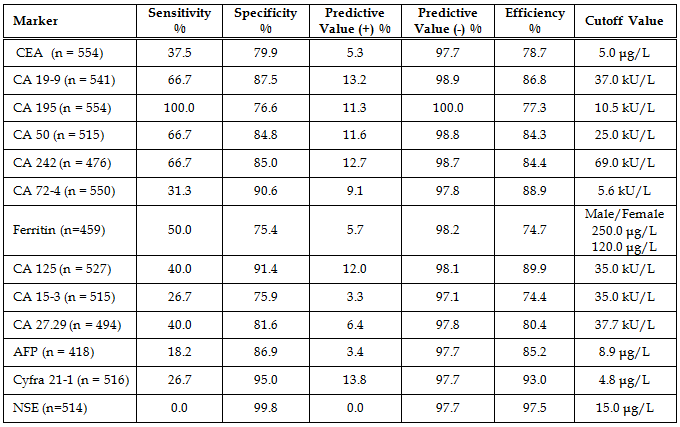
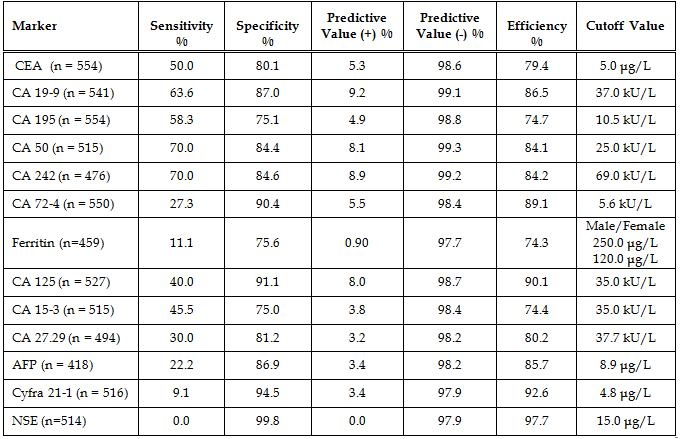
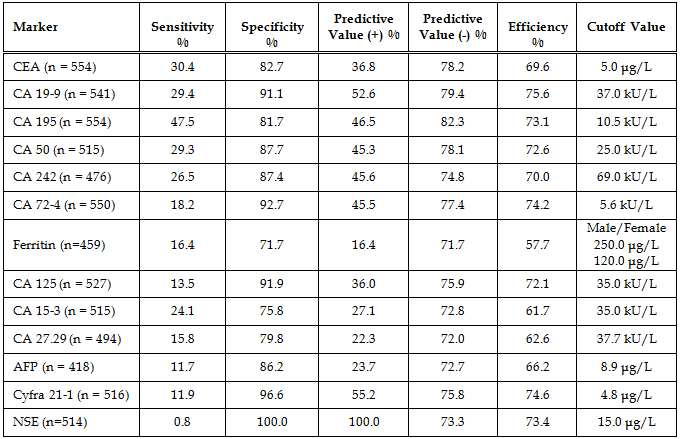
Diagnostic Parameters With the exception of CA242 and ferritin, cutoffs between normal and abnormal test results used in this study were those given by the assay manufacturers and are cited in the table legends. The cutoff used for CA242 was that obtained by our normal reference interval and the ferritin cutoffs for males and females were derived from the literature. The patients’ diagnoses were made by the attending physicians and were predicated on a variety of pathologic findings, including the histologic analysis of biopsy or surgical tissue. In the study there were 184 patients without disease, 11 patients with non-malignant disease, 16 patients with pancreatic cancer, 12 patients with gastric cancer, 101 patients with colorectal cancer, and 230 patients with other types of cancer. The other types of cancer included: 2 esophageal, 3 small intestinal, 3 gallbladder, 4 hepatic, 3 cecal, 17 lung, 87 breast, 6 ovarian, 2 uterine, 17 prostatic, 20 testicular, 6 renal, 6 head and neck, 13 leukemia, 16 lymphoma, and 25 all other types. A comparison of assay results for the pancreatic cancer patients is given in Table 6. The most important finding was that 100% of the patients with pancreatic cancer had abnormally elevated serum CA 195. Especially noteworthy was the fact that 9/16 pancreatic cancer patients had a serum CA 195 concentration which was greater than 20x the upper limit of normal (ULN). Seven of these patients had CA 195 concentrations that were greater than 50 x ULN and two patients had values that were greater than 1000 x ULN prior to their diagnosis by conventional methods (imaging and biopsy). Serum concentrations of all the other tumor antigens were less than 20x ULN in the pancreatic cancer patients. A comparison of assay results for the gastric cancer patients is given in Table 7. Seventy percent of the CA 242 and CA 50 assay results and 63.6% of the CA 19-9 results were elevated in the gastric cancer patients with serum levels reaching 15x ULN for these assays. For CA 195 there were only 7/12 (58.3%) abnormally elevated assay results. However, four of these patients had serum CA 195 concentrations that were greater than 100x ULN. Predictive values were calculated for pancreatic cancer (Table 8), gastric cancer (Table 9), and combined gastrointestinal cancer (Table 10). Disease prevalence for the patient population was 2.89% for pancreatic cancer, 2.17% for gastric cancer, and 25.99% for combined gastrointestinal cancers. The number of patients tested varied according to the volume of sample available and is given in the tables. As a consequence of this, there were minor variations in the disease prevalence for the samples on which each analyte was tested (pancreatic cancer 2.63-3.04%, gastric cancer 1.94-2.17%, combined gastrointestinal cancer 25.82-26.94%). Table 8 shows that the diagnostic sensitivities of CA 195 (100%), CA 19-9 (66.7%), CA 50 (66.7%), and CA 242 (66.7%) were superior to those of the other assays (18.2-50.0%) for pancreatic cancer. In Table 9, the diagnostic sensitivities of CA 50 (70.0%), CA 242 (70.0%), CA 19-9 (63.6%), and CA 195 (58.3%) were superior to those of the other markers (9.1-50%) for gastric cancer. Table 10 gives the predictive values for combined gastrointestinal cancers and reflects the predominance of colorectal cancer patients. The diagnostic sensitivity was less than 50% in each of the assays for combined gastrointestinal cancer (Table 10). These values were similar to those calculated for colorectal cancer (data not shown). The diagnostic specificities of the thirteen assays ranged from 72 - 100% with NSE having the highest value for pancreatic, gastric, and combined gastrointestinal cancers (Tables 8-10). All the assays gave negative predictive values greater than 97% for pancreatic and gastric cancer (Tables 8-9) and between 72% and 82% for combined gastrointestinal cancer (Table 10). Positive predictive values were uniformly low (<14%) for pancreatic and gastric cancer (Tables 8-9), reflecting the fact that there were other cancers which gave positive results. Positive predictive values for combined gastrointestinal cancer (Table 10) were somewhat higher (22-100%). The efficiency was greater than 74% (range 74-98%) in all of the assays for both pancreatic and gastric cancer, presumably due to their high diagnostic specificities (Tables 8-9). In combined gastrointestinal cancers the efficiency ranged from 58% to 76% (Table 10). None of the assays detected the two cases of esophageal cancer.
Discussion In this study, we compared thirteen serologic antigens (CEA, CA 19-9, CA 195, CA 50, CA 242, CA 72-4, ferritin, CA 125, CA 15-3, CA 27.29, AFP, Cyfra 21-1, and NSE) for their efficacy at detecting pancreatic, gastric, and combined gastrointestinal cancer. Analytical parameters compared favorably for all the assays except ferritin. Both the within-run and the between-run precisions were poor for ferritin, but all other values were below 20%. The linearity was excellent for all the assays. The minimum detectable concentration of analyte (zero calibrator/diluent mean + 2SD) was slightly higher for CA 125, ferritin, and NSE than for the other assays. These tests were therefore repeated using patient samples that had previously given a result of 0 kU/L [U/mL] (data not shown). The results did not differ from those of the zero calibrator/diluent, confirming their values. Both the minimum detectable concentration and the normal/healthy adult reference interval for CA 242 were higher than expected. The normal reference intervals were broader than those cited by the manufacturers for all the assays except CA 125, CA 72-4, CA 27.29, AFP, ferritin and NSE. The CA 19-9 assay exhibited a significantly higher reference interval for males than for females; otherwise there were no significant differences between the sexes. The assays compared favorably for cost and availability of instrumentation. With the exception of CEA, CA 242, AFP, ferritin, and NSE, all of the assays were radiolabeled (I125) and therefore had shorter shelf lives. The turnaround time varied from 1 hour for AFP (automated assay) to approximately 3-24 hours for the other assays (manual assays with varying incubation periods). In order to compare the diagnostic parameters of the thirteen tumor antigens, sera from 554 patients seen in a local hospital were assayed and their diagnostic parameters compared. The physicians’ diagnoses and the manufacturers’ suggested cutoff values or cutoff values derived from our normal reference interval (CA 242) and the literature (ferritin) were utilized to assign the test results to the categories of true or false positives and negatives. Predictive values were calculated for pancreatic, gastric, and combined gastrointestinal cancer. The two most important findings of this study were the observations that: (a) CA 195 exhibited 100% diagnostic sensitivity for pancreatic cancer with values reaching 1200x ULN, and (b) CA 50 and CA 242 were clearly superior to CA 72-4 for the detection of gastric cancer, exhibiting diagnostic sensitivities of 70% as compared to 27%. The importance of the pancreatic cancer findings derives from the fact that 9/16 patients exhibited serum CA 195 levels in excess of 20x ULN, seven of these results were greater than 50x ULN and two of these exceeded 1000x ULN prior to patient diagnosis by conventional means. This leads one to wonder if the patients had been tested earlier, might they have been diagnosed sooner when their prognoses were better. The importance of the gastric cancer results stems from the fact that CA 72-4 has been reported to be the best tumor marker for gastric cancer and is currently being marketed as a gastric/gastrointestinal cancer marker. However, our test results suggest that eight other antigens (CA 50, CA 242, CA 19-9, CA 195, CEA, CA 15-3, CA 125, and CA 27.29) were superior (30-70% sensitivity) to CA 72-4 (27% sensitivity) for the detection of gastric cancer. CA 19-9, CA 195, CA 50, and CA 242 exhibited the best diagnostic sensitivities for pancreatic, gastric, and combined gastrointestinal cancers, with CEA performing nearly equivalently for gastric and combined gastrointestinal cancers. Since CA 19-9, CA 195, CA 50, and CA 242 share very similar epitopes, it should not be surprising that all four react similarly. Likewise, CEA shares some antigenic determinants with CA 19-9 (5). By contrast, the diagnostic specificities of CA 72-4, CA 125, Cyfra 21-1, and NSE were superior to those of the other markers for all of the different gastrointestinal cancers. This could be the result of the low prevalence of ovarian and uterine cancer, since three of the markers have been described in cancer of the female reproductive organs (sources for increased false positives and therefore decreased diagnostic specificity). Similarly the low prevalence of cancers of neuroendocrine origin may contribute to the high NSE specificity. The prevalence of lung cancer was also relatively low which could account for the high diagnostic specificities of Cyfra 21-1 and CEA (5, 41). While CA 15-3 has been reported in cases of gastrointestinal cancer (5), in this study it was primarily elevated in cases of breast cancer (63% sensitivity, 81% specificity, 34% PV+, 93% PV-, 78% efficiency for breast cancer). This supports its current use in therapeutic monitoring of mammary cancer patients and explains its modest sensitivity and specificity for gastrointestinal cancers. The combined use of multiple tumor markers is generally believed to increase the sensitivity and decrease the specificity of the test (5). The increased sensitivity is due to the heterogeneity of many tumors with different proportions of their cell populations, and hence of antigens shed by them, being recognized by different assays. The decreased specificity is due to the fact that each assay will give a positive test for some benign and nonmalignant diseases and the use of multiple assays increases the likelihood of detecting elevations of at least one marker in a specimen. Our study results did not support the use of multiple markers for either pancreatic or gastric cancer (data not shown). It should also be noted that there is always the possibility that patients classified as “without disease” may have as yet undiagnosed subclinical disease (cancer). It is conceivable that in the future the use of ratios of multiple tumor markers may allow one to detect a very early cancer and to better discriminate its source. If that should prove to be the case, then it may justify the additional cost of multiple testing. The findings of this study with respect to pancreatic cancer markers are supported by the work of Andicoechea et al., who found CA 195 to be superior to CEA for the diagnosis of pancreatic carcinoma (42). In similar studies, Banfi et al (43) and Giulianotti et al (44) reported that CA 19-9 and CA 195 had equivalent diagnostic sensitivities and these were considerably greater than those for CEA. Banfi also reported that CA 242 had a lower sensitivity but higher specificity than CA 19-9 and CA 195. Masson et al (45) reported diagnostic sensitivities and specificities in excess of 80% for CA 19-9, CA 50, and CA 195, whereas CEA had low specificity when using cutoffs that gave comparable sensitivity. They also observed significant differences in the CA 50 levels detected by two different analytical methods (IRMA vs DELPHIA) using the same monoclonal antibody. In a study by Oremek et al (46), the diagnosic sensitivities of CA 19-9 (68%), CA 50 (63%), CA 72-4 (49%) were superior to CEA (37%) but inferior to a pyruvate kinase-type tumor M2 marker. By contrast, Sagar et al (47) found that both CEA and CA 195 detected pancreatic cancer and the recurrence of disease following surgery. They reported that in patients with metastatic pancreatic cancer, the CA 195 was significantly higher but did not discriminate between operable and inoperable disease. For the diagnosis of gastric cancer, Pectasides et al. (48) found CA 50 and CA 19-9 to be superior to CEA. In a similar study, Haglund et al. investigated CA 19-9 and CA 50 for their diagnostic capabilities and found them to have the same sensitivity for gastric cancer (49). In two other studies, the authors reported a discrepancy between the markers depending on the stage of the cancer. In a study involving 100 cancer patients, Kodama et al. (50) reported that in advanced cancer CA 72-4 was superior to CEA and CA 19-9 for the diagnosis, prognosis, and detection of recurrent disease. By contrast, they found CA 19-9 and CEA to be better for the detection of early stage (I and II) disease. Likewise, in a study by Van-Dalen and Kessler (51) in which serum samples from 23 labs were analyzed for CEA, CA 15-3, CA 19-9, CA 72-4, CA 125, Cyfra 21-1, and AFP, the authors reported that CA 72-4 was the most sensitive for stage IV disease. However, the authors found CA 72-4, CA 19-9, and CEA to be equally sensitive for stage I-III disease. By contrast, in a study of 242 patients by Spila et al. (52), the authors found that CA 72-4 was superior to both CEA and CA 19-9 for the diagnosis and prognosis of both primary and recurrent gastric cancer. Likewise, Fernandez-Fernandez et al. have reported that in a study of 167 patients with gastric cancer and 92 patients with benign disease, they found CA 72-4 to be superior to both CA 19-9 and CEA at all stages of disease (53). Discrepancies between their results and ours could be the result of genetic differences in the patient populations, the stage of the tumors, the presence of pathologic complications and/or the use and type(s) of therapies. Conclusions In conclusion, thirteen assays (CEA, CA 19-9, CA 195, CA 50, CA 242, CA 72-4, ferritin, CA 125, CA 15-3, CA 27.29, AFP, Cyfra 21-1and NSE) were evaluated for their efficacy at diagnosing pancreatic, gastric, and combined gastrointestinal cancer. CA 195, CA 19-9, CA 50, and CA 242 were superior to the other assays for the detection of pancreatic cancer, but only CA 195 detected all of the cases. Likewise, CA 50 and CA 242 proved to be superior to the other assays for gastric cancer with CA 19-9, and CA 195, also proving effective. In contrast to previous studies, our results did not support the use of CA 72-4 for the diagnosis of gastric cancer. None of the assays detected the two cases of esophageal cancer, and none were particularly sensitive for combined gastrointestinal cancer or for colorectal cancer, which constituted the bulk (101/144) of the gastrointestinal cases in our patient population. Acknowledgments The authors thank Ms Jan Oglesby (Kessler AFB), Ms Rebecca Newsome (Puckett Laboratory), Ms Cynthia Wilson (University of Mississippi Medical Center), Ms Kay Holifield (Laurel Clinic for Women), Dr. Charlton Vincent (Laurel Clinic for Women), Mr Joe Acosta (World Freight International Freight Forwarders, Inc.), Mr. Allen Keely (Fujirebio Diagnostics Inc.), and Ms Marcia Muller (Diagnostic Automation, Inc) for their assistance . This study was partially supported by the Aubrey Keith and Ella Ginn Lucas Research Award. Fujirebio Diagnostics Inc./Centocor Inc., Hybritech Inc., Hybritech Europe, CIS bio international, Diagnostic Automation, Inc., and Abbott Laboratories donated reagents. The gift of sera from Keesler AFB Medical Center and Forrest General Hospital is gratefully acknowledged. References
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||