|
Introduction All processors have been plagued for half a century by gel particles and imperfections in plastic-molded and extruded materials. Studies on this topic have demonstrated that the causes were inherent in the molecular structure of each polymer. In early days whole shipments (190,000 pounds, for example) to a processor had been rejected and in some cases came close to bankrupting the supplier. Some times these rejected materials were simply an error in judgment. For example, most people knew that ferric chloride was a catalyst for the decomposition of PVC. However, in 1967 a shipment of PVC, colored orange as required by the wire and cable industry, was received at Buffalo by Western Electric from an approved supplier.Within fifteen minutes after the extrusion was started, the material burned up in the extruder. An analysis of the pigment turned out to be ferric oxide; the PVC, though heavily stabilized, still evolved enough HCl to convert the ferric oxide into ferric chloride, and the results were devastating. A 4 ½ inch extruder used for jacketing large amounts of pairs of cables (25 to be exact) was fitted with a new extruder screw and ran perfectly for a few weeks. Fortunately, I was assigned to monitor that unit and realized that the new screw was changing during the operation and actually wore out completely in five months. The flight clearance went from the new value of 5 mils (the distance between the flight and the barrel) to a value of 30 mils. During this wearing time, I studied the performance of that screw and found that the only way that I could maintain the output was to keep lowering the temperature on the barrel. The conclusion was that as the barrel wore, the degree of shear on the PVC compound increased, and hence, the heat of shear rose dramatically. Thus, the wear on the flights was directly related to some change in the molecular structure of the plastic, causing a possible change in the quality of the polymeric extrudate. Initially, it was thought that the only role of the plastic was to convey the filler, calcium carbonate (particle size of 3 mils in diameter), into the space between the flight and the barrel, and hence, causing the wear. However, we studied the rheological effects on the polymer to see if there might be a change in the plastic itself. The tests using infrared spectroscopy [1], solubility [1], as well as all forms of rheology [2], all indicated a gross change in plastic, suggesting that the molecular structure might be altered. Further tests were run or devised to detect any changes in the basic polymer [3]. The plasticizer was extracted using diethyl ether. The evaporated extract was not pure plasticizer, but contained significant amounts of low molecular weight PVC fragments [4]. In addition 200,000 kilograms of jacketing PVC was recycled to find out whether any of it was reusable. Only 10,000 kilograms made acceptable jackets. The rest had so many gel particles on the surface that it had to be rejected. These gel particles were tested and found to be high molecular weight PVC, estimated to be well over a million in weight average molecular weight (Mw) [5]. Furthermore, it was determined that with semi-rigid or even flexible compounds such as those used in jacketing, could only be extruded four times without displaying gels and imperfections in the final product. To emphasize the active nature of this system, a literature search indicated that this mechano-chemical chain-scission process has been studied extensively by R. J. Ceresa [6]. He indicates that the free radicals are stable enough to be used during mastication for making block and graph copolymers of PVC. This means that not only copolymers can be formed, but also the reactions within PVC itself can be rampant and capable of converting relatively linear PVC into more complex structures and even the precursors of gels [7, 8, 9, 10]. Shear Measurements Shear occurs when one surface moves relative to another surface. The speed of this movement converts that operation into a shear rate. This is very important because excessive shearing effect is the primary cause of breaking molecular bonds of polymers. The relationship between speed and shear rate can be seen in the simple expression Shear rate = ▫ = 4q/▫r3 where q = output in mm3/sec and r = mm. [11] As the output goes up, the shear rate rises directly. Related to this, the heat of shear rises as the square of the shear rate (G = J (dv/dr)2), which is why the shearing function is so effective for melting the polymer in the screw. The shear rates of testing and processing range from a level of 10-3 all the way to 107 reciprocal seconds. The meaning behind all these ranges is as follows:
Test Procedure The All the useful range of rheological testing has been explored completely. The Rheometric Scientific instruments like the Mechanical Spectrometer are significant for identifying molecular structural changes [14]. However, they have not served our needs for observing fast changes in the process during extrusion or related processes. Capillary rheometry can relate to the higher shear rates and directly to the changes in processing steps such as the melting and shearing in the extruder screw. Here the rheology of the testing relates only to the average viscosity at the particular shear rate and only demonstrates gross viscosity changes as a measure of non-uniformity of density and viscosity and, possibly, temperature [15]. Certainly, to define extruder screws and their effective shapes, these capillary viscosities are the best way to attain computer simulation [16]. The molten viscosities are an extension of the unmelted coefficient of friction used so effectively to design complete extruder screws by the process know as Extrud [16]. However, none of these techniques define the manner in which a polymer structure changes effectively as the process alters the material. Furthermore, the analysis requires some judgment of the non-uniformity of the sample through the test procedure. Thus, the real analysis of the sample depends on a long die to attain equilibrium flow, and the ability to get many specimens to show the sensitivity and uniformity of the test data [17]. This has been developed from a modification of the extrusion plastometer [18]. Surprisingly enough, this simple machine has allowed us to detect molecular structural changes in PVC, initially, because the technique runs at such low shear rates and allows equilibrium flow. Then, to a lesser degree, changes can be sensed significantly in polypropylene, polybutylene, and even high-density polyethylene. A complete study of most of the polyolefins is being explored [19]. This technique is widely applicable and is even being applied to polycarbonate and ABS as illustrated effectively in Table 1 [20].
Note how the values for each reading vary, but give a good average. Note how much higher the failed sample of ABS is compared with the control. This means that appreciable fragmenting is occurring in the process. (Care is taken to avoid the early and late cuts that are at non-equilibrium conditions.) Note, too, that the temperature of the test is at 2300C. The “normal” melt flow by ASTM D 1238 for polycarbonate is run at 3000C. Lower temperatures enhance the sensitivity of the method for detecting molecular structural changes. Interestingly enough, the technique has not demonstrated differences in the low-density polyethylene, so far, probably because the molecular weight distribution is so broad. This is being looked into with special highly branched side chains. A complete analytical study was performed on the samples in order to study and define altered flow in the extrusion plastometer. The conclusions arrived at were that the ASTM D 3364 was the best technique for detecting fragmentation and instability in most polymer systems. The analytical systems covered were:
Techniques that will better define the fragments and the more complex molecular structures are being explored. Processing Examples Instability Studies Using Extruders The melt flow rates rose from the original. Note that the melt flow values of the pellets rise to a high at 10 RPM, a clear example of the shearing causing the polymer to be fragmented. At higher RPMs, the melt flow falls sharply, indicating a re-assembly of the free radicals into a more complex structure. Note that the flow rates fall far below the original pellet values, indicating that an “incipient crosslinking” is forming. Excessively complex structures will be discussed later. The process of shearing and fusing PVC compounds to form pellets for subsequent extrusion can alter the material significantly. If the pellets are prepared in slow speed equipment (or in a low shear mixer), the melt flow of the resulting material is expected to be higher than the starting material. If the pellets are made in high-speed equipment, the flow rates will be lower than that of the dry blend and subsequent processing will need higher energy requirements and, in some cases, not extrudable. Demonstrating the Change in Molecular Structure in Compounding Operations This illustrates that in processing, the rheology of molten PVC and other sensitive polymers can be controlled, but not by means normally observed or used in industry. Care must be taken to regulate the rate of shear in the process and to monitor that function, either by means of sensitive instruments associated with the extruder or by carefully controlled design of the extruding equipment. Relationship Between Melt Flow and Amperage for Screw of a 150mm Extruder Having installed a recording amperage meter on a 152.4 mm (6 inch) 20/1 L/D extruder, any appreciable change in the drive motor (440 v.) due to the change in the melt strength of the basic polymer was able to sense. In this case, a uniform pair of samples of flexible jacketing PVC [containing 35 pounds per hundred of resin (phr) of filler—calcium carbonate of 3 micron grind, 60 phr of DOP plasticizer] were used. The PVC Melt Flow by the ASTM Standard gave a value of 300 mg/min and 350 mg/min. The latter sample was loaded into the hopper and the equilibrium amperage of the screw gave a value of 170 amps. This was followed by a hopper load of the 300 melt flow material. The amperage rose to 173, indicating that a difference of 50 mg/min in melt flow is equivalent to 3 amps of difference on the extruder drive motor [1]. Techniques Controlling the Effects of Shear Despite the fact that rigid PVC is inherently prone to shear damage, the proper use of lubricants has proven to be effective in preventing this shear. A complete study of this factor can be found in the literature [13]. If the plasticizer content is raised toward the 50% level (i.e., 90 to 110 phr) the PVC is protected from change by even levels of shear observed in an extruder screw with a compression ratio as high as 15 to 1. A semi-rigid material was tried in this screw and immediately burned up. Size Exclusion Chromatography Results The flow rate increase with fragmentation has been observed also by Pinette [5] in 1990 during processing on a two-roll mill. A series of experiments demonstrated the increase in fragmentation and then, subsequently, a decrease toward crosslinking. To analyze the results, size exclusion chromatography and inherent viscosity measurements were employed on the samples in progressive order of testing. The results are given in Table 2. For details on methods and techniques see references [13] and [14].
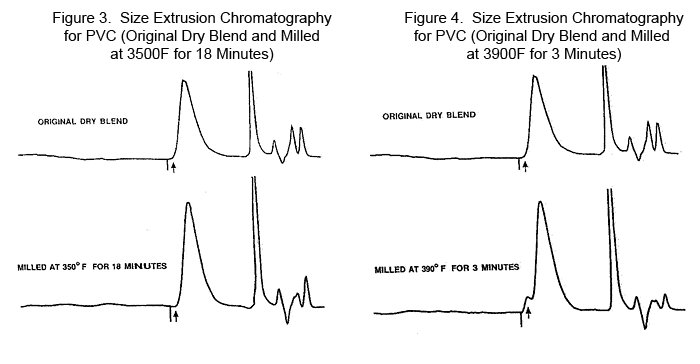
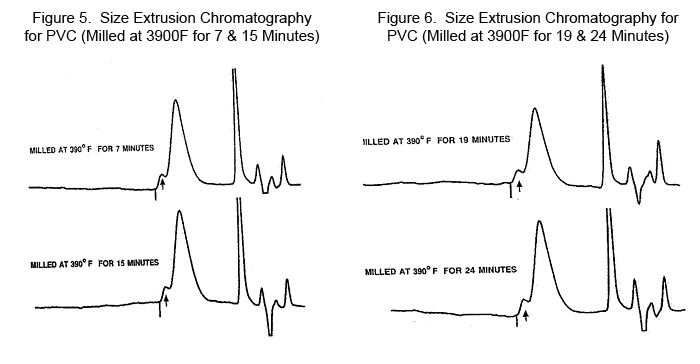
The molecular weights as listed are Number Average Molecular Weight designated as Mn. This molecular weight form is attained by Osmosis and counts the molecular weight based only on the number of end groups of the polymer. The Weight Average Molecular Weight, designated as Mw, is attained by Size Exclusion Chromotograpy. This later term is the most widely used expression and closely and easily related to the international term as the K factor and the Inherent Viscosity. The extrusion process uses the Inherent Viscosity in the range of 0.9 to 1.1 for semi-rigid to flexible PVC and the K factor of 65 to 70. Note that fragmentation of sample 2 at 18 minutes at 3500F gave a melt flow rate of 625 mg/min (up from 118 mg/min starting material) and was observed to have a 16% decrease in weight average molecular weight (Mw). This represents the high point in the melt flow rate curve observed in the data by D 3364. In contrast, the Mw increases very rapidly at 3900F with increasing time of shear. This appears to be moving in the crosslinking direction. Further testing substantiates this point. Note how the dispersion (Mw/Mn) increases. Instead of a value of 3 or less, the values now jump to 6, a real measure of the increase in complexity of the structure. Based on the original PVC at 111,000 for an Mw, the final structure is almost three times as large. New Molecular Structure Detected The size exclusion chromatography measurements for the material milled by Pinette at 1900C displayed an early elution peak as a well-defined shoulder on the side of the primary PVC elution peak. This peak is not evident at lower temperature work. Based on the analysis, the size of this molecule is near 4 million for its Mw. Figures 3, 4, 5, and 6 show size extrusion chromatography for the original dry blend and milled PVC samples at various temperatures and different time intervals.
This molecule is a precursor for becoming a gel in the system. From the table of chromatography and inherent viscosity data one can see that the effect of this complex molecule increases the Mw for the entire sample from the original 111,000 to a value close to 300,000. The concentration of this complex molecule is estimated to be in the range of 5% of the total sample. Interestingly enough, the same size sample was identified from a film of PVC under entirely different processing conditions. Both were exposed to oxygen, probably intensifying the complexity of the molecular structure in that 5% portion of the system. Summary Shearing effects have long been an unrecognized hindrance to proper processing. With the major work in the last fifty years being in the area of heat stabilization, shear changes have now become the major detriment to consistent properties in most polymers. The ranking of shear instability for many polymers has been evaluated and it could be concludet that apparent shear stability ranks in the following order:
The shear effects proceed through a mechanism of fragmentation to a series of re-alignments into a progressively more complex series of structures in PVC. These complex structures are defined as incipient crosslinking. This complex polymeric system has been observed widely in the processing world. However, the results are greatly modified depending on the level of sensitivity to alteration by mechano-chemical chain reactions. Acknowledgements This paper was presented at the August 2005 meeting of the American Chemical Society. Samples for the analyses reported in figures 3, 4, 5 and 6 were prepared by Roger Pinette. References
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
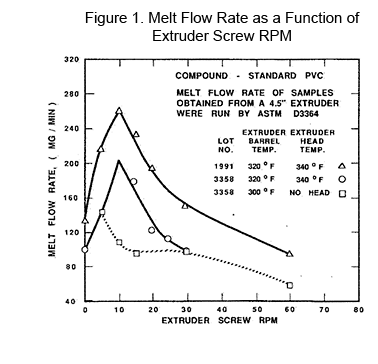 In Figure 1, the instability of melt flow is given as a function of RPM of a 114.3 mm (4 ½'') diameter extruder screw vs. Melt Flow Rate. Three lots of flexible PVC were run through the extruder at normal processing temperatures (1700C) and at different screw speeds. The material was sampled coming out of the crosshead for two cases. A third set of samples was taken directly off the tip of the screw. The differences demonstrate what happens for both the effect of the crosshead vs. the screw alone. The barrel settings ranged from 1500C to 1600C, trying to take heat out of the compound. The head was set at 1700C.
In Figure 1, the instability of melt flow is given as a function of RPM of a 114.3 mm (4 ½'') diameter extruder screw vs. Melt Flow Rate. Three lots of flexible PVC were run through the extruder at normal processing temperatures (1700C) and at different screw speeds. The material was sampled coming out of the crosshead for two cases. A third set of samples was taken directly off the tip of the screw. The differences demonstrate what happens for both the effect of the crosshead vs. the screw alone. The barrel settings ranged from 1500C to 1600C, trying to take heat out of the compound. The head was set at 1700C.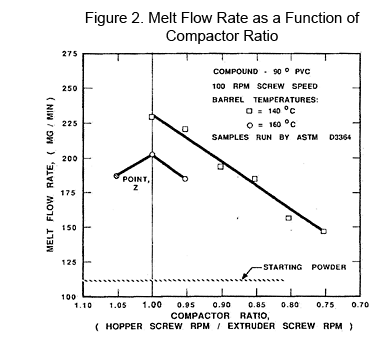 Controlled shear can be demonstrated using a fully instrumented 114.3 mm (4 ½'') screw 28/1 Length/Diameter fitted with a compact feeder (crammer feeder). By increasing the compactor speed between 50% and 100% (from 0.5 to 1.0), the melt flow by ASTM D 3364 of the resulting pellets increased as the speed increased—totally predictable [1], as indicated in Figure 2. Then, as the compactor went from 1.00 to 1.05, the melt flow decreased slightly indicating that the peak in shear fragmentation had been reached.
Controlled shear can be demonstrated using a fully instrumented 114.3 mm (4 ½'') screw 28/1 Length/Diameter fitted with a compact feeder (crammer feeder). By increasing the compactor speed between 50% and 100% (from 0.5 to 1.0), the melt flow by ASTM D 3364 of the resulting pellets increased as the speed increased—totally predictable [1], as indicated in Figure 2. Then, as the compactor went from 1.00 to 1.05, the melt flow decreased slightly indicating that the peak in shear fragmentation had been reached.